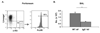IgE influences the number and function of mature mast cells, but not progenitor recruitment in allergic pulmonary inflammation - PubMed (original) (raw)
IgE influences the number and function of mature mast cells, but not progenitor recruitment in allergic pulmonary inflammation
Clinton B Mathias et al. J Immunol. 2009.
Abstract
Studies performed using cultured cells indicate that IgE functions not only to trigger degranulation of mast cells following allergen exposure, but also to enhance their survival. Such an influence of IgE on mast cell homeostasis during allergic responses in vivo has not been established. In this study, we show that inhalation of Aspergillus fumigatus extract in mice induced a dramatic rise in IgE accompanied by an increase in airway mast cells. These had an activated phenotype with high levels of FcepsilonRI. Plasma mast cell protease-1 was also increased, indicating an elevated systemic mast cell load. In addition, enhanced levels of IL-5 and eosinophils were observed in the airway. Both mast cell expansion and activation were markedly attenuated in IgE(-/-) animals that are incapable of producing IgE in response to A. fumigatus. The recruitment of eosinophils to the airways was also reduced in IgE(-/-) mice. Analyses of potential cellular targets of IgE revealed that IgE Abs are not required for the induction of mast cell progenitors in response to allergen, but rather act by sustaining the survival of mature mast cells. Our results identify an important role for IgE Abs in promoting mast cell expansion during allergic responses in vivo.
Figures
Figure 1
Serum IgE levels are elevated in _Af_-treated WT but not IgE−/− mice. Total serum IgE was determined by sandwich ELISA in wild-type (WT) or IgE−/− mice treated with Af or normal saline (NS) for 3 weeks. 8–10 mice were analyzed in each group. nd = none detected, **** = p<0.0001
Figure 2
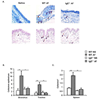
Expansion of mast cells is induced in the trachea, bronchus and spleen of _Af_-treated WT but not IgE−/− mice. WT and IgE−/− mice were treated intranasally with either Af or saline (NS) for 3 weeks and mast cells were enumerated. (A) Toluidine blue (upper panels) and chloroacetate esterase (lower panels) positive mast cells in representative cross-sections of trachea. Mast cells indicated by arrows (red arrow for an intraepithelial mast cell) (B & C) Toluidine blue positive mast cells in the main-stem bronchi, tracheae and spleens of _Af_-treated animals. n=5 mice per group; * = p<0.05; ** = p<0.01; *** = p<0.001
Figure 3
Airway mast cells, enumerated by flow-cytometry, are increased in WT vs. IgE−/− _Af_-treated mice. Peritoneal lavage and BAL fluid from _Af_-treated mice were examined by flow cytometry. (A) Confirmation of the mast cell phenotype of Lineage (Lin)− c-Kit+ peritoneal cells. Cells were stained with lineage-specific antibodies (CD3, B220, CD4, CD8, Gr-1) and anti-c-Kit. Cells residing in the Lin−c-Kit+ gate, (R2), were analyzed for FcεRI expression. (B). Lin− c-Kit+ mast cells are increased in the BAL of _Af_-treated WT mice. Cells from control NS mice could not be analyzed because inadequate numbers were present in the BAL. n=5 mice per group; * = p<0.05
Figure 4
FcεRI expression on mast cells from Af treated mice is increased in the presence of IgE. Peritoneal and lung cells were isolated from normal saline (NS) or _Af_-treated mice and mast cells were examined by flow cytometry. (A) FcεRI staining on peritoneal mast cells from _Af_-treated (solid histograms) WT and IgE−/− mice is shown overlaid on expression in NS control animals (unfilled histograms) of the same genotypes. The levels of FcεRI were assessed by staining with anti-DNP IgE followed by anti-IgE. (B). Median Fluorescence Intensity (MFI) of FcεRI expression on c-Kit+ cells from collagenase-digested lung tissue in normal saline (NS) or _Af_-treated WT and IgE−/− mice. n=5 mice per group; **** = p<0.0001
Figure 5
_Af_-induced increases in murine mast cell protease-1 levels in _Af_-treated mice are enhanced in the presence of IgE. Sera were isolated from normal saline (NS) or _Af_-treated WT and IgE−/− mice after three weeks of treatment and mMCP-1 levels were quantified by ELISA. n=5 mice per group. nd = none detected **** = p<0.0001.
Figure 6
BAL IL-5 levels and IL-5 expression by cultured mast cells and mast cells in vivo are regulated by IgE antibodies. (A) BAL fluid was obtained from normal saline (NS) or _Af_-treated WT and IgE−/− mice and the levels of IL-5 were determined by sandwich ELISA. (B) Bone-marrow derived mast cells were cultured in the presence of medium or 10 µg/ml IgE for 60 min. IL-5 mRNA was measured using real time quantitative PCR. IgG was used as a control. (C) Peritoneal cells were isolated from NS or _Af_-treated WT and IgE−/− mice and number of IL-5-positive cells assessed by intracellular staining.. * = p<0.05; ** = p<0.01; *** = p<0.001.
Figure 7
IgE antibodies regulate the pulmonary cellular infiltrate elicited by allergen inhalation. Differential cell counts were performed on Wright-Geimsa-stained cytocentrifuged BAL specimens from wild-type (WT) and IgE−/− mice treated with Af or normal saline (NS). Percentages of macrophages (Mac), lymphocytes (lymph), polymorphonuclear neutrophils (PMN) and eosinophils (eosin) were enumerated in individual samples and are depicted as a fraction of the total cell count. n=5 mice per group. ns = not significant * = p<0.05; ** = p<0.01; *** = p<0.001; ****=p<0.0001.
Figure 8
Mast cell progenitor recruitment to the airways of Af –treated mice occurs independently of ambient IgE levels. Lung mononuclear cells were isolated from naïve or _Af_-treated WT and IgE−/− mice and mast cell progenitors (MCp) enumerated by limiting dilution culture of IL-3/SCF cultures of lung mononuclear cells as described in Methods. (A) MCp/million lung mononuclear cells and total numbers of lung MCp are shown. (B) CD45+Lin (CD3, CD4, CD8, B220, CD11b, Gr-1)−CD34+β-7integrin+ lung MCps as assessed by flow cytometry.
Figure 9
IgE supports the survival of differentiated mast cells in vivo and provides an anti-apoptotic signal. (A). WT and IgE−/− mice were treated with Af. CFSE-labeled BMMC were injected (2 × 106 cells) into the peritoneal cavities of individual mice. Peritoneal cells were recovered 1, 3 and 6 days later and CFSE+c-Kit+FcεRI+ mast cells enumerated. Mice that received unlabeled BMMC served as controls. n=5 mice per group. *=p<0.05. (B). BrdU labeled BMMC (1 × 106 cells) were injected into the peritoneum of _Af_-treated, WT and IgE−/− mice. 6 days after transfer, c-Kit+BrdU+ and FcεRI+ cells were enumerated by flow cytometry. n=4 mice per group. (C). CFSE-labeled BMMC were injected into the peritoneal cavities of _Af_-treated WT and IgE− − mice. Six days later, transferred BMMC were recovered and apoptosis assessed by determining surface levels of AnnexinV by flow cytometry. Total numbers of CFSE+c-Kit+AnnexinV+ cells in the peritoneum are shown. *p = <0.05; **p = <0.01.
Similar articles
- Human lung mast cell IL-5 gene and protein expression: temporal analysis of upregulation following IgE-mediated activation.
Jaffe JS, Glaum MC, Raible DG, Post TJ, Dimitry E, Govindarao D, Wang Y, Schulman ES. Jaffe JS, et al. Am J Respir Cell Mol Biol. 1995 Dec;13(6):665-75. doi: 10.1165/ajrcmb.13.6.7576704. Am J Respir Cell Mol Biol. 1995. PMID: 7576704 - IL-3 but not monomeric IgE regulates FcεRI levels and cell survival in primary human basophils.
Zellweger F, Buschor P, Hobi G, Brigger D, Dahinden CA, Villiger PM, Eggel A. Zellweger F, et al. Cell Death Dis. 2018 May 1;9(5):510. doi: 10.1038/s41419-018-0526-9. Cell Death Dis. 2018. PMID: 29724998 Free PMC article. - Signalling mechanisms regulating the activation of human eosinophils by mast-cell-derived chymase: implications for mast cell-eosinophil interaction in allergic inflammation.
Wong CK, Ng SS, Lun SW, Cao J, Lam CW. Wong CK, et al. Immunology. 2009 Apr;126(4):579-87. doi: 10.1111/j.1365-2567.2008.02916.x. Epub 2008 Sep 2. Immunology. 2009. PMID: 18771439 Free PMC article. - The role of human mast cell-derived cytokines in eosinophil biology.
Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G. Shakoory B, et al. J Interferon Cytokine Res. 2004 May;24(5):271-81. doi: 10.1089/107999004323065057. J Interferon Cytokine Res. 2004. PMID: 15153310 Review. - 5. IgE, mast cells, basophils, and eosinophils.
Prussin C, Metcalfe DD. Prussin C, et al. J Allergy Clin Immunol. 2006 Feb;117(2 Suppl Mini-Primer):S450-6. doi: 10.1016/j.jaci.2005.11.016. J Allergy Clin Immunol. 2006. PMID: 16455345 Review.
Cited by
- IL-10 Differentially Promotes Mast Cell Responsiveness to IL-33, Resulting in Enhancement of Type 2 Inflammation and Suppression of Neutrophilia.
Ranjitkar S, Krajewski D, Garcia C, Tedeschi C, Polukort SH, Rovatti J, Mire M, Blesso CN, Jellison E, Schneider SS, Ryan JJ, Mathias CB. Ranjitkar S, et al. J Immunol. 2024 May 1;212(9):1407-1419. doi: 10.4049/jimmunol.2300884. J Immunol. 2024. PMID: 38497670 - Peanut allergen inhibition prevents anaphylaxis in a humanized mouse model.
Alakhras NS, Shin J, Smith SA, Sinn AL, Zhang W, Hwang G, Sjoerdsma J, Bromley EK, Pollok KE, Bilgicer B, Kaplan MH. Alakhras NS, et al. Sci Transl Med. 2023 Feb 8;15(682):eadd6373. doi: 10.1126/scitranslmed.add6373. Epub 2023 Feb 8. Sci Transl Med. 2023. PMID: 36753563 Free PMC article. - A Multi-Center Study of the Prevalence and Characteristics of Eosinophilic Phenotype and High IgE Levels Among Chinese Patients with Severe Asthma.
Ko FW, Wang JKL, Hui DSC, Chan JWM, Cheung PS, Yeung YC, Sin KM, Ip MS. Ko FW, et al. J Asthma Allergy. 2023 Jan 25;16:173-182. doi: 10.2147/JAA.S391970. eCollection 2023. J Asthma Allergy. 2023. PMID: 36721738 Free PMC article. - Utilizing mast cells in a positive manner to overcome inflammatory and allergic diseases.
Zhang Z, Ernst PB, Kiyono H, Kurashima Y. Zhang Z, et al. Front Immunol. 2022 Sep 14;13:937120. doi: 10.3389/fimmu.2022.937120. eCollection 2022. Front Immunol. 2022. PMID: 36189267 Free PMC article. Review. - The Emerging Role of Mast Cells in Response to Fungal Infection.
Yu M, Song XT, Liu B, Luan TT, Liao SL, Zhao ZT. Yu M, et al. Front Immunol. 2021 Jun 3;12:688659. doi: 10.3389/fimmu.2021.688659. eCollection 2021. Front Immunol. 2021. PMID: 34149729 Free PMC article. Review.
References
- Holgate ST. Epithelium dysfunction in asthma. J. Allergy Clin. Immunol. 2007;120:1233–1244. quiz 1245-1236. - PubMed
- Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol. 2003;111:450–463. quiz 464. - PubMed
- Meiler F, Zimmermann M, Blaser K, Akdis CA, Akdis M. T-cell subsets in the pathogenesis of human asthma. Curr. Allergy Asthma Rep. 2006;6:91–96. - PubMed
- Umetsu DT, Dekruyff RH. Immune dysregulation in asthma. Curr. Opin. Immunol. 2006;18:727–732. - PubMed
- Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N. Engl. J. Med. 1991;325:1067–1071. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI054471/AI/NIAID NIH HHS/United States
- R01 AI054471-02/AI/NIAID NIH HHS/United States
- P01 AI 031599/AI/NIAID NIH HHS/United States
- P01 AI031599-130004/AI/NIAID NIH HHS/United States
- R01 AI054471-04/AI/NIAID NIH HHS/United States
- R01-AI054471/AI/NIAID NIH HHS/United States
- P01 AI031599/AI/NIAID NIH HHS/United States
- R01 AI054471-05/AI/NIAID NIH HHS/United States
- R01 AI054471-01/AI/NIAID NIH HHS/United States
- R01 AI054471-03/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases