Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response - PubMed (original) (raw)
Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response
Katharina Brandl et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Here, we describe an N-ethyl-N-nitrosourea (ENU)-induced missense error in the membrane-bound transcription factor peptidase site 1 (S1P)-encoding gene (Mbtps1) that causes enhanced susceptibility to dextran sodium sulfate (DSS)-induced colitis. S1P cleaves and activates cAMP response element binding protein/ATF transcription factors, the sterol regulatory element-binding proteins (SREBPs), and other proteins of both endogenous and viral origin. Because S1P has a nonredundant function in the ATF6-dependent unfolded protein response (UPR), woodrat mice show diminished levels of major endoplasmic reticulum chaperones GRP78 (BiP) and GRP94 in the colon upon DSS administration. Experiments with bone marrow chimeric mice reveal a requirement for S1P in nonhematopoietic cells, without which a diminished UPR and colitis develop.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
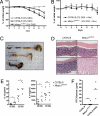
Fig. 1.
The hypopigmented woodrat mutant mice have a point mutation in Mbtps1. (A) The woodrat coat color phenotype at 4 months postpartum. (B) DNA sequence taken from exon 13 of Mbtps1, the gene coding for S1P, in a C57BL/6 WT control (Upper) and a woodrat homozygous mouse (Lower). The mutation is an A-to-G transition that results in a Y-to-C substitution at amino acid 496.
Fig. 2.
Increased susceptibility of woodrat mice to DSS-induced colitis. (A) Percent initial weight of WT and Mbtps1wrt/wrt mice after administration of 2% DSS for 7 days. C57BL/6 mice receiving 0% DSS served as controls. Each group, n = 9–20. *, P < 0.05; **, P < 0.005, 2-tailed Mann–Whitney test for WT and Mbtps1wrt/wrt. (B) C57BL/6 and Mbtps1wrt/wrt were treated with antibiotics in drinking water 5 days before and during the administration of DSS (2%). Weight loss was determined daily, n = 5 each group. (C) Photograph of representative colons and cecums from untreated C57BL/6 mice (top), CD57BL/6 mice treated for 9 days with 2% DSS (middle), and Mbtps1wrt/wrt mice treated for 9 days with 2% DSS (bottom). (D) Representative photomicrographs of colons from WT (C57BL/6) and Mbtps1wrt/wrt mice at days 0 (Upper) and 7 (Lower) of DSS administration. (H&E; magnification: 100×.) (E) Concentrations of IL-1β and IL-6 in supernatants from distal colonic explants cultured for 24 h from WT (C57BL/6) and Mbtps1wrt/wrt mice either before or after 7 days administration of 2% DSS, n = 6–7 each group. *, P < 0.05, Student's t test; n.s., not significant. (F) Plasma FITC-dextran concentrations in WT (C57BL/6) and Mbtps1wrt/wrt mice 4 h following oral gavage (40 mg/100 g body weight) are shown. Mice with damaged epithelial barrier (C57BL/6 mice, receiving 9 days of 2% DSS) served as a positive control, n = 7–8 for WT and Mbtps1wrt/wrt; n = 3 for C57BL/6 with 2% DSS; n.s., not significant.
Fig. 3.
Reduced ER stress response in the colon of woodrat mice after DSS administration. Protein extracts from the distal colon of WT (C57BL/6), Mbtps1+/wrt, and Mbtps1wrt/wrt mice before (A and B) and after (C and D) administration of 2% DSS in the drinking water for 3 days were analyzed by Western blotting (A and C) and quantitative image analysis (B and D) with GRP78- and GRP94-specific antibodies. β-actin was used as a loading control. n = 6–14 (B), n = 11–14 (D). *, P < 0.05; **, P < 0.005, Student's t test for WT and Mbtps1wrt/wrt; n.s., not significant.
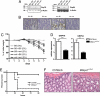
Fig. 4.
ER stress in nonhematopoietic cells correlates with the colitis phenotype. (A) Protein extracts from the distal colon of chimeric mice treated with 2% DSS for 7 days were analyzed by Western blotting with GRP78- and GRP94-specific antibodies. β-Actin was used as a loading control. Shown are representative extracts from each donor–recipient combination (n = 5). (B) Immunohistochemical detection of GRP78 (brown) in paraffin-embedded distal colonic sections in different BM chimeric mice. (C) Weight loss in BM chimeric mice was measured daily upon administration of 2% DSS in the drinking water. C57BL/6 mice receiving 0% DSS served as controls. n = 5 for WT→woodrat (wrt), woodrat→WT; WT→WT and for woodrat→woodrat. Statistical analyses of differences between groups at given days are shown in
Table S6
. (D) mRNA was extracted from colonic epithelial cells from WT (C57BL/6) and Mbtps1wrt/wrt mice. GRP78 and GRP94 expression was examined by quantitative real-time PCR. Expression levels were normalized to β-actin, n = 9–10 each group. **, P < 0.005; ***, P < 0.0005, Student's t test. (E) Tunicamycin was injected i.p. into WT (C57BL/6) and Mbtps1wrt/wrt mice, and survival was monitored for 96 h, n = 11 each group. ***, P < 0.0005, log-rank test. (F) Two representative colonic sections from the distal part of WT and Mbtps1wrt/wrt mice (H&E-stained) are shown. (Magnification: B and F, 200×.)
Similar articles
- ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs.
Ye J, Rawson RB, Komuro R, Chen X, Davé UP, Prywes R, Brown MS, Goldstein JL. Ye J, et al. Mol Cell. 2000 Dec;6(6):1355-64. doi: 10.1016/s1097-2765(00)00133-7. Mol Cell. 2000. PMID: 11163209 - Cartilage-specific ablation of site-1 protease in mice results in the endoplasmic reticulum entrapment of type IIb procollagen and down-regulation of cholesterol and lipid homeostasis.
Patra D, DeLassus E, Liang G, Sandell LJ. Patra D, et al. PLoS One. 2014 Aug 22;9(8):e105674. doi: 10.1371/journal.pone.0105674. eCollection 2014. PLoS One. 2014. PMID: 25147951 Free PMC article. - Analysis of ATF6 activation in Site-2 protease-deficient Chinese hamster ovary cells.
Nadanaka S, Yoshida H, Sato R, Mori K. Nadanaka S, et al. Cell Struct Funct. 2006;31(2):109-16. doi: 10.1247/csf.06015. Epub 2006 Nov 17. Cell Struct Funct. 2006. PMID: 17110786 - Pharmacologic inhibition of S1P attenuates ATF6 expression, causes ER stress and contributes to apoptotic cell death.
Lebeau P, Byun JH, Yousof T, Austin RC. Lebeau P, et al. Toxicol Appl Pharmacol. 2018 Jun 15;349:1-7. doi: 10.1016/j.taap.2018.04.020. Epub 2018 Apr 22. Toxicol Appl Pharmacol. 2018. PMID: 29689241 - A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood.
Brown MS, Goldstein JL. Brown MS, et al. Proc Natl Acad Sci U S A. 1999 Sep 28;96(20):11041-8. doi: 10.1073/pnas.96.20.11041. Proc Natl Acad Sci U S A. 1999. PMID: 10500120 Free PMC article. Review.
Cited by
- Envelope glycoprotein of arenaviruses.
Burri DJ, da Palma JR, Kunz S, Pasquato A. Burri DJ, et al. Viruses. 2012 Oct 17;4(10):2162-81. doi: 10.3390/v4102162. Viruses. 2012. PMID: 23202458 Free PMC article. Review. - Yip1 domain family, member 6 (Yipf6) mutation induces spontaneous intestinal inflammation in mice.
Brandl K, Tomisato W, Li X, Neppl C, Pirie E, Falk W, Xia Y, Moresco EM, Baccala R, Theofilopoulos AN, Schnabl B, Beutler B. Brandl K, et al. Proc Natl Acad Sci U S A. 2012 Jul 31;109(31):12650-5. doi: 10.1073/pnas.1210366109. Epub 2012 Jul 16. Proc Natl Acad Sci U S A. 2012. PMID: 22802641 Free PMC article. - Dietary Green Pea Protects against DSS-Induced Colitis in Mice Challenged with High-Fat Diet.
Bibi S, de Sousa Moraes LF, Lebow N, Zhu MJ. Bibi S, et al. Nutrients. 2017 May 18;9(5):509. doi: 10.3390/nu9050509. Nutrients. 2017. PMID: 28524086 Free PMC article. - Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2-/- mice.
Zhao F, Edwards R, Dizon D, Afrasiabi K, Mastroianni JR, Geyfman M, Ouellette AJ, Andersen B, Lipkin SM. Zhao F, et al. Dev Biol. 2010 Feb 15;338(2):270-9. doi: 10.1016/j.ydbio.2009.12.008. Epub 2009 Dec 16. Dev Biol. 2010. PMID: 20025862 Free PMC article. - Autophagy, microbial sensing, endoplasmic reticulum stress, and epithelial function in inflammatory bowel disease.
Kaser A, Blumberg RS. Kaser A, et al. Gastroenterology. 2011 May;140(6):1738-47. doi: 10.1053/j.gastro.2011.02.048. Gastroenterology. 2011. PMID: 21530740 Free PMC article. Review.
References
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
- Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. - PubMed
- Ye J, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. - PubMed
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous