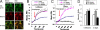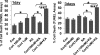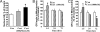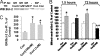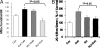Dynamic regulation of mitochondrial function by glucocorticoids - PubMed (original) (raw)
. 2009 Mar 3;106(9):3543-8.
doi: 10.1073/pnas.0812671106. Epub 2009 Feb 6.
Yun Wang, Richard Hunter, Yanling Wei, Rayah Blumenthal, Cynthia Falke, Rushaniya Khairova, Rulun Zhou, Peixiong Yuan, Rodrigo Machado-Vieira, Bruce S McEwen, Husseini K Manji
Affiliations
- PMID: 19202080
- PMCID: PMC2637276
- DOI: 10.1073/pnas.0812671106
Dynamic regulation of mitochondrial function by glucocorticoids
Jing Du et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Glucocorticoids play an important biphasic role in modulating neural plasticity; low doses enhance neural plasticity and spatial memory behavior, whereas chronic, higher doses produce inhibition. We found that 3 independent measures of mitochondrial function-mitochondrial oxidation, membrane potential, and mitochondrial calcium holding capacity-were regulated by long-term corticosterone (CORT) treatment in an inverted "U"-shape. This regulation of mitochondrial function by CORT correlated with neuroprotection; that is, treatment with low doses of CORT had a neuroprotective effect, whereas treatment with high doses of CORT enhanced kainic acid (KA)-induced toxicity of cortical neurons. We then undertook experiments to elucidate the mechanisms underlying these biphasic effects and found that glucocorticoid receptors (GRs) formed a complex with the anti-apoptotic protein Bcl-2 in response to CORT treatment and translocated with Bcl-2 into mitochondria after acute treatment with low or high doses of CORT in primary cortical neurons. However, after 3 days of treatment, high, but not low, doses of CORT resulted in decreased GR and Bcl-2 levels in mitochondria. As with the in vitro studies, Bcl-2 levels in the mitochondria of the prefrontal cortex were significantly decreased, along with GR levels, after long-term treatment with high-dose CORT in vivo. These findings have the potential to contribute to a more complete understanding of the mechanisms by which glucocorticoids and chronic stress regulate cellular plasticity and resilience and to inform the future development of improved therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
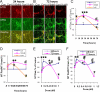
Fig. 1.
Mitochondrial oxidation has an inverted “U”-shaped relationship with CORT dose. Cortical neurons (10–12DIV) were treated with 100 nM, 500 nM, or 1 μM CORT for 1 h, 1 day, or 3 days and stained with MTR and MTG. Data were combined from 2–3 independent experiments and presented as mean ± SE (*one-way ANOVA ***P < 0.001, **P < 0.05,*P < 0.01 compared to control; # P < 0.05 compared to group of 1 μM and 72-hr treatment, n = 32–70). (A) The sample image of MTR and MTG staining after CORT treatment for 24 h. (B) The sample image of MTR and MTG staining after CORT treatment for 72 h. (C) Time course of mitochondrial oxidation by MTR. (D) Time course of mitochondrial oxidation by MTR/MTG ratio. (E) Dose-dependent curve for mitochondrial oxidation by MTR. (F) Dose-dependent curve for mitochondrial oxidation by MTR/MTG ratio.
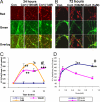
Fig. 2.
CORT modulates membrane potential in a dose- and time-dependent manner. Data were combined from 2–3 independent experiments and presented as mean ± SE (*one-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001 compared to control, #P < 0.01 compared to 72 h and 1 μM CORT treatment, n = 34–90). (A) JC-1 staining image after 24 h treatment with 100 nM and 1 μM of CORT. (B) JC-1 staining image after 72 h treatment with 100 nM and 1 μM of CORT. (C) Time course of JC-1 staining after CORT treatment. (D) Dose-dependent curve for JC-1 staining after CORT treatment.
Fig. 3.
Modulation of mitochondrial calcium holding capacity by CORT in a time- and dose-dependent manner. Data are presented as mean ± SE (one-way ANOVA, n = 4, n = 223. *P < 0.05, **P < 0.01 compared to control, # P < 0.001 compared to the group of CORT 1 μM for 3 days). (A) Rhod-2 colocalizes with MTG in cortical neurons. (B) Mitochondrial calcium levels (Fi/Fo) in response to thapsigargin stimulation in cortical neurons after 1.5-hr treatment with CORT. (C) Mitochondrial calcium levels in response to thapsigargin stimulation in cortical neurons after 3-day treatment with CORT. (D) Comparison of calcium holding capacity 10 mins after thapsigargin stimulation in CORT-treated cortical neurons.
Fig. 4.
The effect of low and high concentrations of CORT in KA-induced apoptosis. Cortical neurons (10–12DIV) were treated with CORT (100 nM, 1 μM) for 1 day in Neurobasal and 3 days in N2 plus Neurobasal. KA (50 μM) was then applied for 12 h to challenge the neurons. Neuronal apoptosis was determined by TUNEL assay (n = 2, n = 17–29 for each group. One-way ANOVA, *P < 0.05, **P < 0.01). Data are presented as mean ± SE.
Fig. 5.
GRs translocate into mitochondria in response to low and high concentrations of CORT. Data were combined from 2–3 independent experiments and presented as mean ± SE. (A) GR translocation into nucleus after CORT treatment after 1.5 h treatment (one-way ANOVA, P < 0.05, n = 18). (B) GR translocation into mitochondria after 100 nM CORT treatment (*Student's t test P < 0.05, n = 8–10). (C) GR translocation into mitochondria after 1 μM CORT treatment (*Student's t test P < 0.05, n = 8–10)
Fig. 6.
Bcl-2 coimmunoprecipitated with GRs and translocated into mitochondria after CORT treatment in a time- and dose-dependent manner. (A) Bcl-2 coimmunoprecipitates with GRs in cultured cortical neurons after 30-min treatment (lane1–3: IP with anti-GR antibody; lane 4: total protein from cortical neurons; lane 5: IP with anti-IGF antibody; lane 6: no antibody). (B) The formation of GR/Bcl-2 complexes was enhanced in cortical neurons after 30-min treatment with CORT (1 μM and 100 nM) (n = 6, n = 5–8, Student's t test P < 0.05). (C) Bcl-2 translocated into mitochondria after CORT treatment in cultured cortical neurons (n = 2–3, n = 8–14, Student's t test, **P < 0.01, *P < 0.05). Data are presented as mean ± SE.
Fig. 7.
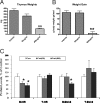
RU486 has an antagonist effect on CORT-induced GR translocation and mitochondrial potential in cortical neurons. (A) RU486 exerted antagonist effects on CORT-induced GR translocation into mitochondria (N = 2–3, n = 56. One-way ANOVA, *P < 0.05). (B) RU486 effect on CORT-induced increase in mitochondrial potential revealed by JC-1 staining (N = 3–4, n = 346. One-way ANOVA, *P < 0.01, **P < 0.05). Data are presented as mean ± SE.
Fig. 8.
GR and Bcl-2 levels in mitochondria are decreased in mitochondrial fractions in prefrontal cortex after chronic stress and after CORT treatment. Western blot analysis of GR and Bcl-2 levels was performed in total homogenates (T) and mitochondrial fractions (M) from prefrontal cortex of CORT (50 μg/ml, 400 μg/ml)-treated animals. Western blot analysis of GR and Bcl-2 content was performed in total homogenates (T) and mitochondrial (M) fractions from prefrontal cortex. Data are presented as mean ± SE. (A) Thymus weights in CORT-treated animals (n = 8 animals per group; **one-way ANOVA, P < 0.01). (B) Body weights of CORT-treated animals (n = 8 animals per group, one-way ANOVA, ** P < 0.01) (C) Chronic CORT treatment significantly reduced mitochondrial GR and Bcl-2 levels (Control group n = 12–15; CORT 50 μg/ml, n = 8; CORT 400 μg/ml, n = 12–15, *one-way ANOVA, P < 0.05)
Similar articles
- Bag-1 mediates glucocorticoid receptor trafficking to mitochondria after corticosterone stimulation: Potential role in regulating affective resilience.
Luo S, Hou Y, Zhang Y, Feng L, Hunter RG, Yuan P, Jia Y, Li H, Wang G, K Manji H, S McEwen B, Xiao C, Bao H, Du J. Luo S, et al. J Neurochem. 2021 Jul;158(2):358-372. doi: 10.1111/jnc.15211. Epub 2020 Oct 21. J Neurochem. 2021. PMID: 33025573 - Long-term continuous corticosterone treatment decreases VEGF receptor-2 expression in frontal cortex.
Howell KR, Kutiyanawalla A, Pillai A. Howell KR, et al. PLoS One. 2011;6(5):e20198. doi: 10.1371/journal.pone.0020198. Epub 2011 May 27. PLoS One. 2011. PMID: 21647420 Free PMC article. - The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels.
Chen C, Nakagawa S, An Y, Ito K, Kitaichi Y, Kusumi I. Chen C, et al. Front Neuroendocrinol. 2017 Jan;44:83-102. doi: 10.1016/j.yfrne.2016.12.001. Epub 2016 Dec 9. Front Neuroendocrinol. 2017. PMID: 27956050 Review. - Glucocorticoids and their receptors: insights into specific roles in mitochondria.
Lee SR, Kim HK, Song IS, Youm J, Dizon LA, Jeong SH, Ko TH, Heo HJ, Ko KS, Rhee BD, Kim N, Han J. Lee SR, et al. Prog Biophys Mol Biol. 2013 May;112(1-2):44-54. doi: 10.1016/j.pbiomolbio.2013.04.001. Epub 2013 Apr 16. Prog Biophys Mol Biol. 2013. PMID: 23603102 Review.
Cited by
- Deep rest: An integrative model of how contemplative practices combat stress and enhance the body's restorative capacity.
Crosswell AD, Mayer SE, Whitehurst LN, Picard M, Zebarjadian S, Epel ES. Crosswell AD, et al. Psychol Rev. 2024 Jan;131(1):247-270. doi: 10.1037/rev0000453. Epub 2023 Dec 25. Psychol Rev. 2024. PMID: 38147050 Free PMC article. - PINK1-mediated mitophagy contributes to glucocorticoid-induced cathepsin K production in osteocytes.
Yuan J, Gao YS, Liu DL, Pang Tai AC, Zhou H, Papadimitriou JM, Zhang CQ, Zheng MH, Gao JJ. Yuan J, et al. J Orthop Translat. 2022 Nov 24;38:229-240. doi: 10.1016/j.jot.2022.11.003. eCollection 2023 Jan. J Orthop Translat. 2022. PMID: 36474855 Free PMC article. - Neuroendocrine Stress Axis-Dependence of Duloxetine Analgesia (Anti-Hyperalgesia) in Chemotherapy-Induced Peripheral Neuropathy.
Staurengo-Ferrari L, Bonet IJM, Araldi D, Green PG, Levine JD. Staurengo-Ferrari L, et al. J Neurosci. 2022 Jan 19;42(3):405-415. doi: 10.1523/JNEUROSCI.1691-21.2021. Epub 2021 Dec 8. J Neurosci. 2022. PMID: 34880120 Free PMC article. - Joining the dots: neurobiological links in a functional analysis of depression.
Sharpley CF, Bitsika V. Sharpley CF, et al. Behav Brain Funct. 2010 Dec 11;6:73. doi: 10.1186/1744-9081-6-73. Behav Brain Funct. 2010. PMID: 21143991 Free PMC article. Review. - Acute Effects of Systemic Glucocorticoids on the Vocal Folds in a Pre-Clinical Model.
Gartling G, Nakamura R, Sayce L, Kimball EE, Wilson A, Schneeberger S, Zimmerman Z, Garabedian MJ, Branski RC, Rousseau B. Gartling G, et al. Ann Otol Rhinol Laryngol. 2024 Jan;133(1):87-96. doi: 10.1177/00034894231188571. Epub 2023 Jul 27. Ann Otol Rhinol Laryngol. 2024. PMID: 37497827 Free PMC article.
References
- Gold PE, McGaugh JL. In: Neuropeptide Influences on the Brain and Behavior. Miller LH, Sandman CA, Kastin AJ, editors. New York: Raven Press; 1977. pp. 127–143.
- Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2005;27:244–249. - PubMed
- Gould E, Woolley CS, McEwen BS. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990;37:367–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH041256/MH/NIMH NIH HHS/United States
- R37 MH041256/MH/NIMH NIH HHS/United States
- Z01 MH002840-04/ImNIH/Intramural NIH HHS/United States
- MH41256/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials