Epigenetic reprogramming and small RNA silencing of transposable elements in pollen - PubMed (original) (raw)
Epigenetic reprogramming and small RNA silencing of transposable elements in pollen
R Keith Slotkin et al. Cell. 2009.
Abstract
The mutagenic activity of transposable elements (TEs) is suppressed by epigenetic silencing and small interfering RNAs (siRNAs), especially in gametes that could transmit transposed elements to the next generation. In pollen from the model plant Arabidopsis, we show that TEs are unexpectedly reactivated and transpose, but only in the pollen vegetative nucleus, which accompanies the sperm cells but does not provide DNA to the fertilized zygote. TE expression coincides with downregulation of the heterochromatin remodeler decrease in DNA methylation 1 and of many TE siRNAs. However, 21 nucleotide siRNAs from Athila retrotransposons are generated and accumulate in pollen and sperm, suggesting that siRNA from TEs activated in the vegetative nucleus can target silencing in gametes. We propose a conserved role for reprogramming in germline companion cells, such as nurse cells in insects and vegetative nuclei in plants, to reveal intact TEs in the genome and regulate their activity in gametes.
Figures
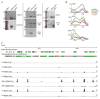
Figure 1. TEs are expressed in mature pollen
Microarray profiles of TEs reveal changes in expression during development. Changes in expression are indicated as up regulation (green), or down regulation (red) compared to the row-normalized expression for each ORF across array experiments (Schmid et al., 2005). The scale (bottom) represents the log2 change over the mean of all values for a given ORF. The scale has been set from −2 to +2 to emphasize small changes in gene expression. The microarray experiment identifiers are listed across the top, as well as their corresponding developmental tissue and stage (AM is apical meristem). Probes and their corresponding ORFs and Repbase annotation are shown on the right. Repbase annotations are indicated as LTR retrotransposons, non-LTR retrotransposons (italic), DNA transposons (bold) and helitron elements (bold and italic). Probe sets are generally unique to an individual ORF. However, probe names that include ‘s’ detect two or more paralogs, while probes with an ‘x’ represent theoretical genes from paralogous groups. Shown on the left is a representation of the short arm of chromosome 1 defining the location and chromatin context for each TE. The centromere is at the bottom, near ORF At1g35995. Gene-rich regions are depicted as thin black lines, while TE-rich heterochromatic regions are black ovals.
Figure 2. TE expression is confined to the pollen cytoplasm
(A) GUS staining of non-transgenic L_er_ mature pollen has no staining. (B) GUS staining of the representative GT7390 inserted into an AtCopia LTR retrotransposon has diffuse cytoplasmic GUS staining. (C) DAPI staining of the same pollen grain in (B) shows the location of the vegetative nucleus (VN) and two sperm cells (SCs). (D) Merged images show that the GUS staining does not correspond to the SCs. (E) GT7390 shows no GUS staining in a mature unfertilized carpel. (F) As a positive control, a GT insertion into the PROLIFERA gene (GT148) stained in the ovules. (G–H) Clearing of immature carpels fails to detect GUS staining in the developing megagametophytes for GT7390. Stray pollen grains from GT7390 can be seen in the center of the carpel (G), and serve as a positive control. (I) The control GT148 shows ovule staining post-fertilization, while the representative GT7390 as well as others from Supplemental Table 3 do not show staining of mature ovules post-fertilization (J). (K) GUS staining of flower buds from the representative GT7390 show GUS staining beginning at floral developmental stages 10–11, while L_er_ flower buds do not show GUS staining at this stage (L). (M) GUS staining pollen tubes (arrow) from the representative GT7390 are detected within the carpel of a self-fertilized GT7390 flower.
Figure 3. The mobilization and inheritance of a TE in pollen
(A) Transposable element display was performed under stringent conditions to amplify only AtMu1 family members AtMu1a and AtMu1b. DNA was extracted from either the pooled leaves or pooled pollen from the same 96 Columbia reference strain wild-type plants. These DNA samples are the same as in Supplemental Figure 5. New transpositions were detected in pollen compared to the leaf sample. These new polymorphisms were sequenced and the insertion sites of three new AtMu1a insertions are shown. (B) Transposable element display performed on the progeny of the plants used in part A. If new insertions in pollen were inherited by the next generation, then new insertions would segregate at a low frequency in the self-fertilized progeny of the 96 plants used for pollen collection in part A. The “Col leaf” and “Col pollen” samples are the same samples as in part A. The “Col 8 progeny leaf” and “Col 500 progeny seedling” samples are pools of 8 and 500 individuals that are the self-fertilized progeny of the plants used for leaf and pollen collection in part A. The “Col 500 seedling (spiked)” sample represents 500 Columbia seedlings pooled together with 5 L_er_ seedlings prior to DNA extraction. This sample serves as a positive control for the sensitive detection of rare polymorphisms in a DNA sample. The polymorphisms found in the spiked control are from L_er_, as shown in the “L_er_ leaf” sample (arrows).
Figure 4. DNA methylation of the AtMu1a TE in pollen and sperm
The AtMu1a element (At4g08680) was amplified from bisulfite converted DNA and sequenced from leaf tissue, mature pollen and purified SCs. PCR primers in TE-flanking DNA were used to anchor the analysis to one particular TE copy. The location of the DNA transposon AtMu1 terminal inverted repeat (TIR) is shown as a rectangle, while the flanking DNA (a helitron element into which AtMu1 has inserted) is shown as a line. Twenty-four clones were sequenced for each strand (sense or antisense) of each sample (leaf, pollen or sperm), and only unique “non-sister” individual bisulfite clones are shown. The number of times each unique clone was sequenced is shown next to the clone. The methylation state of each cytosine is shown as an opened circle (not methylated) or closed circle (methylated). The sequence context of the cytosine is denoted by the color of the circle (red = CG, blue = CHG, green = CHH). All cytosines in the DNA sequence are shown. Bisulfite sequencing of a control gene and the AtGP1 retrotransposon are shown in Supplemental Figure 6.
Figure 5. Pollen, sperm and ddm1 siRNAs
A. Northern blots of mature pollen RNA were sequentially hybridized with probes that detect microRNA161 and TE siRNAs. AtGP1 and Athila are LTR retrotransposons, AtSINE2A is a non-autonomous non-LTR retrotransposon, and AtMu1 is a DNA transposon. B. Sequencing of small RNA libraries was used to quantify changes in size distribution and relative abundance for several TE families (AtMu1, Athila and AtGP1) across several samples: wild-type inflorescence (blue), ddm1 inflorescence (green), as well as wild-type pollen (red), and purified sperm (orange) isolated by FACS. The X-axis shows siRNA size (20–26nt), while the Y-axis represents the library size normalized relative number of siRNAs sequenced per TE category (Athila, AtGP1, AtMu1). C. Distributions of 21nt and 24nt siRNAs are shown for a section of pericentromeric heterochromatin from chromosome 4. Red represents DNA transposons, green is retrotransposon, and pink are tandem repeat islands. Bars represent library size-normalized, genome copy-corrected counts of 21 and 24nt siRNA in 100bp windows for wt inflorescence, wt pollen, ddm1 inflorescence, and wt sperm. In pericentromeric heterochromatin, large peaks of 21nt siRNAs in ddm1, pollen and sperm match Athila family retrotransposons.
Figure 6. DDM1 protein accumulation is sperm-specific
GFP reporter lines monitoring DDM1 protein localization were examined by confocal microscopy. The location of the vegetative nucleus (VN) and sperm cells (SCs) were detected in the same pollen grain using DAPI (arrows). The control pollen grain without GFP has only background auto-florescence. GEX2p-GFP control pollen grains have sperm-specific protein accumulation (Engel et al., 2005), while histone H3.3-GFP pollen grains (a gift from Y. Fang and D. Spector) have VN-specific GFP fluorescence. Six independent transformation events revealed sperm-specific protein accumulation of DDM1-GFP. Three-dimensional movies of these pollen grains are included as Supplemental Data.
Figure 7. Model of pollen TE reactivation and potential reprogramming
The pollen VN and SCs (stained here with DAPI) differ in DDM1 localization, TE expression, mobilization and DNA methylation. Reactivated Athila retrotransposons from the VN produce 21nt siRNAs, which accumulate in the SCs. Thus Athila retrotransposons may be “revealed” by epigenetic reprogramming of the VN specifically to target and reinforce their silencing in the SCs.
Similar articles
- Gene expression and stress response mediated by the epigenetic regulation of a transposable element small RNA.
McCue AD, Nuthikattu S, Reeder SH, Slotkin RK. McCue AD, et al. PLoS Genet. 2012 Feb;8(2):e1002474. doi: 10.1371/journal.pgen.1002474. Epub 2012 Feb 9. PLoS Genet. 2012. PMID: 22346759 Free PMC article. - Establishing epigenetic variation during genome reprogramming.
Borges F, Martienssen RA. Borges F, et al. RNA Biol. 2013 Apr;10(4):490-4. doi: 10.4161/rna.24085. RNA Biol. 2013. PMID: 23774895 Free PMC article. - Silencing in sperm cells is directed by RNA movement from the surrounding nurse cell.
Martínez G, Panda K, Köhler C, Slotkin RK. Martínez G, et al. Nat Plants. 2016 Mar 21;2:16030. doi: 10.1038/nplants.2016.30. Nat Plants. 2016. PMID: 27249563 - Reprogramming the epigenome in Arabidopsis pollen.
Borges F, Calarco JP, Martienssen RA. Borges F, et al. Cold Spring Harb Symp Quant Biol. 2012;77:1-5. doi: 10.1101/sqb.2013.77.014969. Epub 2013 Apr 25. Cold Spring Harb Symp Quant Biol. 2012. PMID: 23619015 Free PMC article. Review. - Small RNAs in pollen.
He H, Yang T, Wu W, Zheng B. He H, et al. Sci China Life Sci. 2015 Mar;58(3):246-52. doi: 10.1007/s11427-015-4800-0. Epub 2015 Jan 29. Sci China Life Sci. 2015. PMID: 25634522 Review.
Cited by
- Fundamentally different repetitive element composition of sex chromosomes in Rumex acetosa.
Jesionek W, Bodláková M, Kubát Z, Čegan R, Vyskot B, Vrána J, Šafář J, Puterova J, Hobza R. Jesionek W, et al. Ann Bot. 2021 Jan 1;127(1):33-47. doi: 10.1093/aob/mcaa160. Ann Bot. 2021. PMID: 32902599 Free PMC article. - Reconstructing de novo silencing of an active plant retrotransposon.
Marí-Ordóñez A, Marchais A, Etcheverry M, Martin A, Colot V, Voinnet O. Marí-Ordóñez A, et al. Nat Genet. 2013 Sep;45(9):1029-39. doi: 10.1038/ng.2703. Epub 2013 Jul 14. Nat Genet. 2013. PMID: 23852169 - Apomixis in plant reproduction: a novel perspective on an old dilemma.
Barcaccia G, Albertini E. Barcaccia G, et al. Plant Reprod. 2013 Sep;26(3):159-79. doi: 10.1007/s00497-013-0222-y. Epub 2013 Jul 14. Plant Reprod. 2013. PMID: 23852378 Free PMC article. Review. - From correlation to causation: The new frontier of transgenerational epigenetic inheritance.
Rothi MH, Greer EL. Rothi MH, et al. Bioessays. 2023 Jan;45(1):e2200118. doi: 10.1002/bies.202200118. Epub 2022 Nov 9. Bioessays. 2023. PMID: 36351255 Free PMC article. - Stable unmethylated DNA demarcates expressed genes and their cis-regulatory space in plant genomes.
Crisp PA, Marand AP, Noshay JM, Zhou P, Lu Z, Schmitz RJ, Springer NM. Crisp PA, et al. Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23991-24000. doi: 10.1073/pnas.2010250117. Epub 2020 Sep 2. Proc Natl Acad Sci U S A. 2020. PMID: 32879011 Free PMC article.
References
- Aravin AA, Hannon GJ, Brennecke J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science. 2007;318:761–764. - PubMed
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–1103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources