Small molecule blockers of the Alzheimer Abeta calcium channel potently protect neurons from Abeta cytotoxicity - PubMed (original) (raw)
Small molecule blockers of the Alzheimer Abeta calcium channel potently protect neurons from Abeta cytotoxicity
Juan Carlos Diaz et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Alzheimer's disease (AD) is a common, chronic neurodegenerative disease that is thought to be caused by the neurotoxic effect of the Amyloid beta peptides (Abeta). We have hypothesized that the intrinsic Abeta calcium channel activity of the oligomeric Abeta polymer may be responsible for the neurotoxic properties of Abeta, and that Abeta channel blockers may be candidate AD therapeutics. As a consequence of a rational search paradigm based on the model structure of the Abeta channel, we have identified two compounds of interest: MRS2481 and an enatiomeric species, MRS2485. These are amphiphilic pyridinium salts that both potently block the Abeta channel and protect neurons from Abeta toxicity. Both block the Abeta channel with similar potency (approximately 500 nM) and efficacy (100%). However, we find that inhibition by MRS2481 is easily reversible, whereas inhibition by MRS2485 is virtually irreversible. We suggest that both species deserve consideration as candidates for Alzheimer's disease drug discovery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
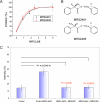
Inhibition of Aβ neurotoxicity by MRS2485 and MRS2481. (A) Titration of protective effects of MRS2485 and MRS2481. The ED50 values for both compounds are ≈500 nM. PC12 cells were cultured to near confluence, and then incubated with fresh medium and 5 μM Aβ for 3 days. Cell survival was measured using a colorimetric XTT assay. Similar results were obtained with LDH release (not shown). All experiments were repeated independently on at least three occasions. (B) Structures of MRS2485 and MRS2481. X− is bromide in this experiment. Toxicity assay is by XTT. Details on synthesis are given by Tchilibon et al. (56). Specific analytic information for each of these compounds is summarized in
Fig. S1
. (C) Inhibition of Aβ-induced calcium uptake by MRS2485 and MRS2481. Cells were exposed to 5 μM Aβ or 5 μM Aβ + MRS2481 or MRS2485, at a saturating drug concentrations of 12 μM. After 2 hours cells were washed, fixed and incubated in medium containing the cell permeant calcium-sensitive dye Calcium Crimson-AM, as described in methods section. Fluorescence levels were measured from 50–60 individual cells, and microscopic digital images analyzed. P values in red show no significant difference from control.
Fig. 2.
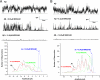
Influence of MRS2481 and MRS2485 on Aβ channel activity in planar lipid bilayers. (A) Traces of Abeta42 channel activity after exposure to MRS2481. The current traces show the channel activity from Aβ incorporated in a planar lipid bilayer. The bilayer is maintained at zero electrical membrane potential and separates two compartments containing asymmetrical concentrations of CsCl (200cis/50trans mM). The amplitude histograms of the channel activity from the current traces show that the drug essentially bring the Aβ channel activity to a virtual complete halt. (B) Discontinuous traces of Aβ channel activity after exposure to MRS2485. Planar lipid bilayer experiments were performed exactly as described for Fig. 3_A._ Histograms show that the drug essentially halted the Aβ channel activity.
Fig. 3.
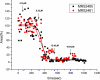
Conductance versus time and drug concentration for MRS2485 (black squares) and MRS2881 (red balls). Data show the total ionic current flowing through the Aβ channel incorporated into the artificial lipid membrane at any given time. For this purpose, following the time course of the Aβ channel activity, we integrated the total ionic current flowing through the membrane and averaged the amount of charge conducted in consecutive time intervals of 8 milliseconds' duration. The integration was initiated after the incorporated channel had achieved stable activity and also after the addition of the test compounds. The area (%) is the normalized total ionic current flowing through the Aβ channel incorporated into the artificial lipid membrane over an 8-second period. The highest and lowest area current values are as depicted. Drugs are added systematically at the time shown by the asterisk symbols on the horizontal time axis. The arrows in the body of the graph indicate the cumulative drug concentrations. Each addition is 4.16 μM. There is a precipitous drop in the ionic current flowing through the Aβ channel after the drug concentrations reaches 8.32 μM. After the lower level of ionic current area is reached, bursts of channel activity permit some ionic current to flow through the Aβ channel bathed with MRS2481. By contrast, a sustained block of channel activity is noted for MRS2485. The nature of the two compounds is thus manifest as a “flickery block” for MRS2481. The curves are calculated as power functions [y = VMAX * x n/(kn + xn)]. R2 values for MRS2481 and MRS2485 are 0.87 and 0.86, respectively. The values of the slopes of these curves varies somewhat: n = −6.44 ± 1.00 for MRS2481 and n = −9.48 ± 1.93 for MRS2485.
Fig. 4.
Influence of “washout” on retention of Aβ channel blocking activity by MRS2485 versus MRS2481 in a planar lipid bilayer. (A) Influence of washout on MRS2481 Aβ channel blocking activity. MRS2481 is applied to an active Aβ channel, and observed to completely block the channel. After washing the drug out of the chamber with three successive volume replacements, recovery of full channel activity is observed. (B) Influence of washout on MRS2485 Aβ channel blocking activity. MRS2485 is applied to an active Aβ channel and is observed to completely block the channel. After washing the drug out of the chamber with three successive volume replacements, channel activity is not recovered.
Similar articles
- Inhibiting Aβ toxicity in Alzheimer's disease by a pyridine amine derivative.
Zhu Z, Yang T, Zhang L, Liu L, Yin E, Zhang C, Guo Z, Xu C, Wang X. Zhu Z, et al. Eur J Med Chem. 2019 Apr 15;168:330-339. doi: 10.1016/j.ejmech.2019.02.052. Epub 2019 Feb 20. Eur J Med Chem. 2019. PMID: 30826509 - Effects of amyloid-β peptides on voltage-gated L-type Ca(V)1.2 and Ca(V)1.3 Ca(2+) channels.
Kim S, Rhim H. Kim S, et al. Mol Cells. 2011 Sep;32(3):289-94. doi: 10.1007/s10059-011-0075-x. Epub 2011 Aug 4. Mol Cells. 2011. PMID: 21822937 Free PMC article. - Neurotoxicity of β-amyloid protein: oligomerization, channel formation, and calcium dyshomeostasis.
Kawahara M. Kawahara M. Curr Pharm Des. 2010;16(25):2779-89. doi: 10.2174/138161210793176545. Curr Pharm Des. 2010. PMID: 20698821 Review. - Mechanistic Insight into the Design of Chemical Tools to Control Multiple Pathogenic Features in Alzheimer's Disease.
Han J, Du Z, Lim MH. Han J, et al. Acc Chem Res. 2021 Oct 19;54(20):3930-3940. doi: 10.1021/acs.accounts.1c00457. Epub 2021 Oct 4. Acc Chem Res. 2021. PMID: 34606227 Review.
Cited by
- β-Barrel topology of Alzheimer's β-amyloid ion channels.
Jang H, Arce FT, Ramachandran S, Capone R, Lal R, Nussinov R. Jang H, et al. J Mol Biol. 2010 Dec 17;404(5):917-34. doi: 10.1016/j.jmb.2010.10.025. Epub 2010 Oct 21. J Mol Biol. 2010. PMID: 20970427 Free PMC article. - Misfolded amyloid ion channels present mobile beta-sheet subunits in contrast to conventional ion channels.
Jang H, Arce FT, Capone R, Ramachandran S, Lal R, Nussinov R. Jang H, et al. Biophys J. 2009 Dec 2;97(11):3029-37. doi: 10.1016/j.bpj.2009.09.014. Biophys J. 2009. PMID: 19948133 Free PMC article. - Differences between amyloid-β aggregation in solution and on the membrane: insights into elucidation of the mechanistic details of Alzheimer's disease.
Kotler SA, Walsh P, Brender JR, Ramamoorthy A. Kotler SA, et al. Chem Soc Rev. 2014 Oct 7;43(19):6692-700. doi: 10.1039/c3cs60431d. Chem Soc Rev. 2014. PMID: 24464312 Free PMC article. Review. - Endogenous Amyloid-formed Ca2+-permeable Channels in Aged 3xTg AD Mice.
Li S, Ji X, Gao M, Huang B, Peng S, Wu J. Li S, et al. Function (Oxf). 2023 May 26;4(4):zqad025. doi: 10.1093/function/zqad025. eCollection 2023. Function (Oxf). 2023. PMID: 37342418 Free PMC article. - Misfolded protein oligomers induce an increase of intracellular Ca2+ causing an escalation of reactive oxidative species.
Fani G, La Torre CE, Cascella R, Cecchi C, Vendruscolo M, Chiti F. Fani G, et al. Cell Mol Life Sci. 2022 Aug 27;79(9):500. doi: 10.1007/s00018-022-04513-w. Cell Mol Life Sci. 2022. PMID: 36030306 Free PMC article.
References
- Ashe KH. Mechanisms of memory loss in Aβ and tau mouse models. Biochem Soc Trans. 2005;33:591–594. - PubMed
- German DC, Nelson O, Liang F, Liang CL, Games D. The PDAPP mouse model of Alzheimer's disease: Locus coeruleus neuronal shrinkage. J Comp Neurol. 2005;492:469–476. - PubMed
- Glenner GG, Wong CW. Alzheimer's disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Comm. 1984;120:885–890. - PubMed
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, et al. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–776. - PubMed
- Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987;235:877–880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK031127/DK/NIDDK NIH HHS/United States
- R37 DK031127/DK/NIDDK NIH HHS/United States
- R56 DK031127/DK/NIDDK NIH HHS/United States
- Z01 DK031127/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials