Prenatal programming of sexual partner preference: the ram model - PubMed (original) (raw)
Review
Prenatal programming of sexual partner preference: the ram model
C E Roselli et al. J Neuroendocrinol. 2009 Mar.
Abstract
In our laboratory, the domestic ram is used as an experimental model to study the early programming of neural mechanisms underlying same-sex partner preference. This interest developed from the observation that approximately 8% of domestic rams are sexually attracted to other rams (male-oriented) in contrast to the majority of rams that are attracted to oestrous ewes (female-oriented). One prominent feature of sexual differentiation in many species is the presence of a sexually dimorphic nucleus (SDN) in the preoptic/anterior hypothalamus that is larger in males than in females. Lesion studies in rats and ferrets implicate the SDN in the expression of sexual preferences. We discovered an ovine SDN (oSDN) in the preoptic/anterior hypothalamus that is smaller in male- than in female-oriented rams and similar in size to the oSDN of ewes. Neurones of the oSDN show abundant aromatase expression that is also reduced in male-oriented compared to female-oriented rams. This observation suggests that sexual partner preferences are neurologically hard-wired and could be influenced by hormones. Aromatase-containing neurones constitute a nascent oSDN as early as day 60 of gestation, which becomes sexually dimorphic by day 135 of gestation when it is two-fold larger in males than in females. Exposure of fetal female lambs to exogenous testosterone from days 30-90 of gestation resulted in a masculinised oSDN. These data demonstrate that the oSDN develops prenatally and may influence adult sexual preferences. Surprisingly, inhibition of aromatase activity in the brain of ram foetuses during the critical period did not interfere with defeminisation of adult sexual partner preference or oSDN volume. These results fail to support an essential role for neural aromatase in the sexual differentiation of sheep brain and behaviour. Thus, we propose that oSDN morphology and male-typical partner preferences may instead be programmed through an androgen receptor mechanism not involving aromatisation.
Figures
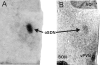
Figure 1
Coronal view through the medial preoptic area-anterior hypothalamus showing the location of the oSDN. (A) Digitised autoradiogram illustrating aromatase mRNA expression. (B) Thionin stained adjacent brain section. Abbreviations: AC, anterior commissure; SON, supraoptic nucleus; vPVN, ventral paraventricular nucleus. Reprinted with permission from (23)
Figure 2
Differences in the volume of the oSDN among luteal phase ewes and gonadally-intact female- and male-oriented rams. Data are presented as means ± SEM. Bars marked with different letters are significantly different; P < 0.05. Reprinted with permission from (42).
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.
Ryan R, Hill S. Ryan R, et al. Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article. - Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.
Love AMA, Edwards C, Cai RY, Gibbs V. Love AMA, et al. Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article. - "I've Spent My Whole Life Striving to Be Normal": Internalized Stigma and Perceived Impact of Diagnosis in Autistic Adults.
Huang Y, Trollor JN, Foley KR, Arnold SRC. Huang Y, et al. Autism Adulthood. 2023 Dec 1;5(4):423-436. doi: 10.1089/aut.2022.0066. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116050 Free PMC article. - The neurobiology of sexual partner preferences in rams.
Roselli CE, Stormshak F. Roselli CE, et al. Horm Behav. 2009 May;55(5):611-20. doi: 10.1016/j.yhbeh.2009.03.013. Horm Behav. 2009. PMID: 19446078 Free PMC article. Review.
Cited by
- Developmental and Functional Effects of Steroid Hormones on the Neuroendocrine Axis and Spinal Cord.
Zubeldia-Brenner L, Roselli CE, Recabarren SE, Gonzalez Deniselle MC, Lara HE. Zubeldia-Brenner L, et al. J Neuroendocrinol. 2016 Jul;28(7):10.1111/jne.12401. doi: 10.1111/jne.12401. J Neuroendocrinol. 2016. PMID: 27262161 Free PMC article. Review. - Effects of Long-Term Flutamide Treatment During Development on Sexual Behaviour and Hormone Responsiveness in Rams.
Roselli CE, Meaker M, Stormshak F, Estill CT. Roselli CE, et al. J Neuroendocrinol. 2016 May;28(5):10.1111/jne.12389. doi: 10.1111/jne.12389. J Neuroendocrinol. 2016. PMID: 27005749 Free PMC article. - Programmed for Preference: The Biology of Same-Sex Attraction in Rams.
Roselli CE. Roselli CE. Neurosci Biobehav Rev. 2020 Jul;114:12-15. doi: 10.1016/j.neubiorev.2020.03.032. Epub 2020 Apr 18. Neurosci Biobehav Rev. 2020. PMID: 32311371 Free PMC article. Review. - Complex actions of estradiol-3-sulfate in late gestation fetal brain.
Winikor J, Schlaerth C, Rabaglino MB, Cousins R, Sutherland M, Wood CE. Winikor J, et al. Reprod Sci. 2011 Jul;18(7):654-65. doi: 10.1177/1933719110395400. Epub 2011 Jan 27. Reprod Sci. 2011. PMID: 21273638 Free PMC article. - Sexual partner preference in animals and humans.
Balthazart J. Balthazart J. Neurosci Biobehav Rev. 2020 Aug;115:34-47. doi: 10.1016/j.neubiorev.2020.03.024. Epub 2020 May 22. Neurosci Biobehav Rev. 2020. PMID: 32450091 Free PMC article. Review.
References
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. - PubMed
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:253–286. - PubMed
- Adkins-Regan E, Mansukhani V, Thompson R, Yang S. Organizational actions of sex hormones on sexual partner preference. Brain Res Bull. 1997;44:497–502. - PubMed
- Baum MJ. Mammalian animal models of psychosexual differentiation: When is ‘translation’ to the human situation possible? Horm Behav. 2006;50:579–588. - PubMed
- Bakker J. Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. J Neuroendocrinol. 2003;15:615–621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources