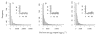Density-dependent effects on the weight of female Ascaris lumbricoides infections of humans and its impact on patterns of egg production - PubMed (original) (raw)
Density-dependent effects on the weight of female Ascaris lumbricoides infections of humans and its impact on patterns of egg production
Martin Walker et al. Parasit Vectors. 2009.
Abstract
Background: Ascaris lumbricoides exhibits density-dependent egg production, a process which has a marked impact on both the transmission dynamics and the stability of the parasite population. Evidence suggests that the egg production of female Ascaris is also associated with the size of the worm. If worm size is mediated by density-dependent processes then the size of female worms may have a causal impact upon patterns of Ascaris egg production.
Results: We analyse data collected from a cohort of human hosts, and demonstrate that the per host mean weight (a proxy for size) of female Ascaris is dependent on the number of infecting females (worm burden) following a pattern of initial facilitation followed by limitation. Applying a negative binomial (NB) generalized linear model (GLM) and a zero-inflated negative binomial (ZINB) model we confirm that the per host female mean weight is significantly associated with per host egg production. Despite these associations, the mean weight of female Ascaris has little causal impact on patterns of density-dependent egg output. The ZINB model is able to account for the disproportionately large number of zero egg counts within the data and is shown to be a consistently better fit than the NB model. The probability of observing a zero egg count is demonstrated as being negatively associated with both female worm burden and female mean weight.
Conclusion: The mean weight of female Ascaris is statistically significantly associated with egg output, and follows a consistent pattern of facilitation preceding limitation with increasing female worm burden. Despite these relationships, incorporation of female Ascaris mean weight into models of egg output has little effect on patterns of density dependence. The ZINB model is a superior fit to the data than the NB model and provides additional information regarding the mechanisms that result in a zero egg count. The ZINB model is shown to be a useful tool for the analysis of individual-based egg output data.
Figures
Figure 1
The relationship between the per host net egg output and mean weight of female Ascaris lumbricoides. The relationship between the per host net egg output and the (centred, see main text) mean weight of female Ascaris in the baseline (A), 1st (B) and 2nd (C) re-infection populations. Triangles represent grouped mean egg outputs stratified by female Ascaris mean weight. Solid lines and circles represent the fitted values of the best fit polynomial functions (as determined by the likelihood-ratio statistic (LRS), see Table 3).
Figure 2
The distribution of per host egg output. Histograms depicting the distribution of the per host egg output in the baseline (A), 1st (B) and 2nd (C) re-infection populations. The insets are histograms of the distribution between 0–100 eggs gram-1 highlighting the high proportion of zero counts.
Figure 3
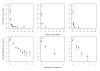
Relationship between the proportion of zero egg counts and female worm burden. Top row: a scatter plot of the proportion of zero egg counts per stratum of female Ascaris worm burden in the baseline (A), 1st (B) and 2nd (C) re-infection populations. Bottom row: logit of the proportion of zero egg counts per stratum of the natural logarithm of female worm burden in the baseline (D), 1st (E) and 2nd (F) re-infection populations showing approximately linear relationships.
Figure 4
Relationship between the proportion of zero egg counts and the mean weight of female Ascaris lumbricoides. Top row: a scatter plot of the proportion of zero egg counts per stratum of female Ascaris mean weight in the baseline (A), 1st (B) and 2nd (C) re-infection populations. Bottom row: logit of the proportion of zero egg counts per stratum of the natural logarithm of female mean weight baseline (D), 1st (E) and 2nd (F) re-infection populations showing approximately linear relationships.
Figure 5
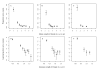
The best-fit relationship between the per host mean weight of female Ascaris lumbricoides and female worm burden. The best-fit functional relationships (as determined by the LRS, Table 6) between the per host mean weight of female Ascaris and the female worm burden in the baseline (A), 1st (B), and 2nd (C) re-infection populations. The solid red line is the best-fit to children (age ≤ 12 years) and the broken blue line to teenagers and adults (age > 12 years). The best-fit function is given by equation (2) and represents a pattern of initial facilitation followed by limitation. Circular and square data points are grouped means for children and teenagers and adults respectively. Error bars represent the standard error of the mean.
Figure 6
Comparison of the fit of a log-normal and zero-truncated negative binomial model. A: The estimated variance-to-mean relationship from the zero-truncated negative binomial model (black thick line) and the log-normal model (black thin line). B: The fitted zero-truncated (thick lines) and log-normal (thin lines) models to data from children (red solid line) and teenagers and adults (blue broken line) in the baseline population. In both figures red circles represent grouped mean data from children and blue squares from teenagers and adults (as defined in Figure 5). Details of the variance-to-mean relationship for the log-normal and zero-truncated negative binomial models are given in additional file 1.
References
- Anderson RM, May RM. Infectious Diseases of Humans. Oxford: Oxford University Press; 1992.
- Anderson RM, May RM. Regulation and stability of host-parasite population interactions I. Regulatory processes. Journal of Animal Ecology. 1978;47:219–247.
- Churcher TS. Modelling the Spread of Anthelmintic Resistance. PhD thesis. Imperial College London, Department of Infectious Disease Epidemiology; 2006.
- Churcher TS, Basáñez M-G. Density dependence and the spread of anthelmintic resistance. Evolution. 2008;62:528–537. - PubMed
- Churcher TS, Filipe JAN, Basáñez M-G. Density dependence and the control of helminth parasites. Journal of Animal Ecology. 2006;75:1313–1320. - PubMed