Structural and functional analysis of the interaction between the nucleoporin Nup214 and the DEAD-box helicase Ddx19 - PubMed (original) (raw)
Structural and functional analysis of the interaction between the nucleoporin Nup214 and the DEAD-box helicase Ddx19
Johanna Napetschnig et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Key steps in the export of mRNA from the nucleus to the cytoplasm are the transport through the nuclear pore complex (NPC) and the subsequent remodeling of messenger RNA-protein (mRNP) complexes that occurs at the cytoplasmic side of the NPC. Crucial for these events is the recruitment of the DEAD-box helicase Ddx19 to the cytoplasmic filaments of the NPC that is mediated by the nucleoporin Nup214. Here, we present the crystal structure of the Nup214 N-terminal domain in complex with Ddx19 in its ADP-bound state at 2.5 A resolution. Strikingly, the interaction surfaces are not only evolutionarily conserved but also exhibit strongly opposing surface potentials, with the helicase surface being positively and the Nup214 surface being negatively charged. We speculate that the positively charged surface of the interacting ADP-helicase binds competitively to a segment of mRNA of a linearized mRNP, passing through the NPC on its way to the cytoplasm. As a result, the ADP-helicase would dissociate from Nup214 and replace a single bound protein from the mRNA. One cycle of protein replacement would be accompanied, cooperatively, by nucleotide exchange, ATP hydrolysis, release of the ADP-helicase from mRNA and its rebinding to Nup214. Repeat of these cycles would remove proteins from a mRNP, one at a time, akin to a ratchet mechanism for mRNA export.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
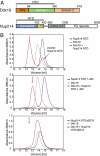
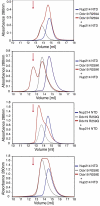
The 6D7A loop of the Nup214 NTD is essential for Ddx19 binding. (A) Domain organization of Nup214 and Ddx19. For Ddx19, the N-terminal extension (NTE, orange) and the 2 RecA-like domains (domain 1 and 2, green and light orange) are indicated. For Nup214, the β-propeller domain (light blue) and its C-terminal extension (CTE, yellow), the coiled-coil domain (gray), and the C-terminal unstructured region containing numerous FG-repeats (white) are indicated. The Nup214 NTD is composed of the β-propeller domain followed by the CTE. The bars above the domain structures mark the fragments of the orthorhombic crystal form. (B) Gel filtration profiles of full-length wild-type Ddx19 incubated with the Nup214 NTD, Nup214 NTD 1–405, or Nup214 NTD Δ6D7A before injection.
Fig. 2.
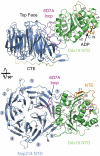
Overview of the Nup214 NTD·Ddx19 NTD structure. Ribbon representation of the Nup214 NTD·Ddx19 NTD complex (Upper). A 90° rotated view is shown in Lower. For the Nup214 NTD, the β-propeller domain (blue), the 6D7A loop (magenta), the C-terminal extension (CTE; yellow), and the blade numbers are indicated. For the Ddx19 NTD, the N-terminal RecA-like domain (green) and the unique N-terminal extension (NTE; orange) is indicated. The ADP molecule bound to the Ddx19 NTD is shown in ball-and-stick representation.
Fig. 3.
Surface properties of the Nup214 NTD-Ddx19 NTD interaction. (A) Surface renditions of the NTDs of Nup214 and Ddx19 in an open book representation colored according to the participation of the various domains as in Fig. 2. Surfaces that mediate the association between the 2 proteins are indicated in green (Ddx19) and blue (Nup214). As a reference, a ribbon representation of the complex is shown in its original orientation. (B) Surface representation colored according to a multispecies sequence alignment (
Fig. S1
). The conservation at each position is mapped onto the surface and is shaded in a color gradient from light yellow (60% similarity) to dark red (100% identity). (C) Surface representation colored according to the electrostatic potential. The electrostatic potential is plotted onto the surface and colored in a gradient from red (−10 k_B_T/e) to blue (+10 k_B_T/e). The orientation of all surface representations is identical. Black lines indicate the interface borders.
Fig. 4.
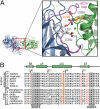
The conserved arginine 259 of Ddx19 is a key residue for complex formation. (A) Details of the interaction between the NTDs of Nup214 and Ddx19. The ribbon representation is colored according to Fig. 2. The Inset illustrates the position of R259 and its interacting residues and is expanded on the right. (B) Multispecies sequence alignment of Ddx19 homologs. The red asterisk indicates the location of the invariant R259. The conserved sequence motifs II and III are highlighted in gray boxes. Invariant k_B_T residues outside of the conserved sequence motifs and R259 are illustrated in red. The residue numbering is relative to human Ddx19 and the secondary structure of Ddx19 is shown above the sequence alignment.
Fig. 5.
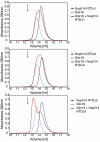
A 9-residue region in the 6D7A loop of the Nup214 NTD is essential for Ddx19 binding. Gel filtration profiles of full-length wild-type Ddx19 incubated with the deletion mutants Nup214 NTDΔ1, Δ2, or Δ3 before injection. Gel filtration profiles of Ddx19 are colored in gray, the Nup214 NTD variants in blue, and the elution profile resulting from incubating Ddx19 with Nup214 proteins in red. The red arrow indicates the expected elution volume of the Nup214·Ddx19 complex (see Fig. 1_B Top_).
Fig. 6.
Arginine 259 of Ddx19 is crucial for binding to Nup214 NTD. Gel filtration profiles of the Nup214 NTD (blue), the Ddx19 mutants R259A, R259Q, R259K and R259E (gray), and the elution profile resulting from incubation of Nup214 NTD with Ddx19 (red) before injection are indicated. The red arrow indicates the expected elution volume of the Nup214·Ddx19 complex (see Fig. 1_B Top_).
Fig. 7.
In vivo localization of Ddx19 and Ddx19 mutants. HeLa cells were transfected with Ddx19 and Ddx19 mutants containing a C-terminal HA-tag and analyzed with confocal microscopy. The monoclonal antibody mAb414 was used as a reference for nuclear envelope staining (red). The cellular localization of HA-tagged proteins was detected with an anti-HA antibody (green). The merged image reveals the colocalization of wild type Ddx19 and Ddx19 R259K with the nuclear envelope, while Ddx19 R259A displays no detectable nuclear envelope staining.
Similar articles
- The mRNA export protein DBP5 binds RNA and the cytoplasmic nucleoporin NUP214 in a mutually exclusive manner.
von Moeller H, Basquin C, Conti E. von Moeller H, et al. Nat Struct Mol Biol. 2009 Mar;16(3):247-54. doi: 10.1038/nsmb.1561. Epub 2009 Feb 15. Nat Struct Mol Biol. 2009. PMID: 19219046 - Structural and functional analysis of mRNA export regulation by the nuclear pore complex.
Lin DH, Correia AR, Cai SW, Huber FM, Jette CA, Hoelz A. Lin DH, et al. Nat Commun. 2018 Jun 13;9(1):2319. doi: 10.1038/s41467-018-04459-3. Nat Commun. 2018. PMID: 29899397 Free PMC article. - Mechanistic insights into mRNA export through structures of Dbp5.
Ling SH, Song H. Ling SH, et al. RNA Biol. 2010 Jan-Feb;7(1):23-7. doi: 10.4161/rna.7.1.10576. Epub 2010 Jan 9. RNA Biol. 2010. PMID: 20023400 - Dbp5, Gle1-IP6 and Nup159: a working model for mRNP export.
Folkmann AW, Noble KN, Cole CN, Wente SR. Folkmann AW, et al. Nucleus. 2011 Nov-Dec;2(6):540-8. doi: 10.4161/nucl.2.6.17881. Epub 2011 Nov 1. Nucleus. 2011. PMID: 22064466 Free PMC article. Review. - Dbp5 - from nuclear export to translation.
Tieg B, Krebber H. Tieg B, et al. Biochim Biophys Acta. 2013 Aug;1829(8):791-8. doi: 10.1016/j.bbagrm.2012.10.010. Epub 2012 Nov 2. Biochim Biophys Acta. 2013. PMID: 23128325 Review.
Cited by
- Control of mRNA export and translation termination by inositol hexakisphosphate requires specific interaction with Gle1.
Alcázar-Román AR, Bolger TA, Wente SR. Alcázar-Román AR, et al. J Biol Chem. 2010 May 28;285(22):16683-92. doi: 10.1074/jbc.M109.082370. Epub 2010 Apr 6. J Biol Chem. 2010. PMID: 20371601 Free PMC article. - Crystal structure of the N-terminal domain of Nup358/RanBP2.
Kassube SA, Stuwe T, Lin DH, Antonuk CD, Napetschnig J, Blobel G, Hoelz A. Kassube SA, et al. J Mol Biol. 2012 Nov 9;423(5):752-65. doi: 10.1016/j.jmb.2012.08.026. Epub 2012 Sep 7. J Mol Biol. 2012. PMID: 22959972 Free PMC article. - Mechanisms of nuclear mRNA export: A structural perspective.
Xie Y, Ren Y. Xie Y, et al. Traffic. 2019 Nov;20(11):829-840. doi: 10.1111/tra.12691. Epub 2019 Sep 12. Traffic. 2019. PMID: 31513326 Free PMC article. Review. - NUP214 in Leukemia: It's More than Transport.
Mendes A, Fahrenkrog B. Mendes A, et al. Cells. 2019 Jan 21;8(1):76. doi: 10.3390/cells8010076. Cells. 2019. PMID: 30669574 Free PMC article. Review. - 8 Å structure of the outer rings of the Xenopus laevis nuclear pore complex obtained by cryo-EM and AI.
Tai L, Zhu Y, Ren H, Huang X, Zhang C, Sun F. Tai L, et al. Protein Cell. 2022 Oct;13(10):760-777. doi: 10.1007/s13238-021-00895-y. Epub 2022 Jan 11. Protein Cell. 2022. PMID: 35015240 Free PMC article.
References
- Hoelz A, Blobel G. Cell biology: Popping out of the nucleus. Nature. 2004;432:815–816. - PubMed
- Cook A, Bono F, Jinek M, Conti E. Structural biology of nucleocytoplasmic transport. Annu Rev Biochem. 2007;76:647–671. - PubMed
- Alcazar-Roman AR, Tran EJ, Guo S, Wente SR. Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nat Cell Biol. 2006;8:711–716. - PubMed
- Weirich CS, et al. Activation of the DExD/H-box protein Dbp5 by the nuclear-pore protein Gle1 and its coactivator InsP6 is required for mRNA export. Nat Cell Biol. 2006;8:668–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials