Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice - PubMed (original) (raw)
Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice
Daniela Tropea et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Rett Syndrome (RTT) is a severe form of X-linked mental retardation caused by mutations in the gene coding for methyl CpG-binding protein 2 (MECP2). Mice deficient in MeCP2 have a range of physiological and neurological abnormalities that mimic the human syndrome. Here we show that systemic treatment of MeCP2 mutant mice with an active peptide fragment of Insulin-like Growth Factor 1 (IGF-1) extends the life span of the mice, improves locomotor function, ameliorates breathing patterns, and reduces irregularity in heart rate. In addition, treatment with IGF-1 peptide increases brain weight of the mutant mice. Multiple measurements support the hypothesis that RTT results from a deficit in synaptic maturation in the brain: MeCP2 mutant mice have sparse dendritic spines and reduced PSD-95 in motor cortex pyramidal neurons, reduced synaptic amplitude in the same neurons, and protracted cortical plasticity in vivo. Treatment with IGF-1 peptide partially restores spine density and synaptic amplitude, increases PSD-95, and stabilizes cortical plasticity to wild-type levels. Our results thus strongly suggest IGF-1 as a candidate for pharmacological treatment of RTT and potentially of other CNS disorders caused by delayed synapse maturation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
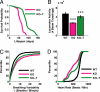
Changes in organismal physiology in MeCP2 mutant mice and the effects of IGF-1 treatment. (A) Lifespan as measured by Kaplan-Meier survival curves, showing the proportion of mice that survived (y axis) at each day after birth (x axis) for nontreated (KO) and treated (KO-T) mice. MeCP2 knockout mice treated with (1–3)IGF-1 daily from P15 onward exhibited a significantly longer life expectancy that their littermates (KO, n = 26 mice; KO-T, n = 21 mice; P < 0.00001, log rank test). (B) Locomotor function, measured by placing animals in cages equipped with infrared beams to quantify nocturnal movement. The y axis shows the number of beam crossings over 10 h in mice aged 8–9 weeks. Compared to wild-type (WT), MeCP2 knockout mice (KO) showed significantly less activity. However, KO mice treated with (1–3)IGF-1 from P15 onward (KO-T) were more active than vehicle-treated KO animals (WT, 46786 ± 15601 beam crossings, n = 17 mice; KO, 27215 ± 6893 crossings, n = 17 mice; KO-T, 40455 ± 21592 crossings, n = 39 mice; WT vs. KO P < 0.00001; KO vs. KO-T P < 0.001, 2-tailed t test). ***: P < 0.001. (C) Breathing variability, assessed by measuring breaths per minute with an oximeter and quantifying the change from one measurement interval (15 seconds) to the next. Comparisons are between 8-week-old mice. MeCP2 KO mice showed increased breathing variability (larger changes per interval) than wild-type littermates (WT, 6.0 ± 0.6 breaths/minute, 630 measurements, n = 13 mice; KO, 15.4 ± 1.1 breaths/minute, 975 measurements, n = 17 mice; P < 0.00001, Kolmogorov-Smirnov test). KO-T mice, treated from P15 for 6 weeks, showed decreased variability (smaller changes per interval) than KO (KO-T, 12.3 ± 0.8 breaths/minute, 1292 measurements, n = 24 mice; KO-T vs. KO P < 0.01, Kolmogorov-Smirnov test). (D) Pooled heart rate distributions observed across mice from different treatments (in beats per minute). Comparisons are between 8-week-old mice (WT, KO, and animals which received (1–3)IGF-1 treatment from P:15 for 6 weeks, KO-T). The KO distribution (pink) was left-shifted compared to the wild-type distribution (black), indicating a significant reduction in the distribution of heart rates (WT, n = 114347 samples, n = 5 mice; KO, 198021 samples, n = 5 mice; P < 0.00001, Kolmogorov-Smirnov test). The KO-T distribution (green) was in-between the two curves (KO-T, 241251 samples, n = 9 mice; KO-T vs. KO P < 0.00001, Kolmogorov-Smirnov test), indicating a partial rescue of the KO phenotype toward a more normal wild-type distribution.
Fig. 2.
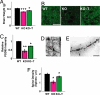
Changes in brain structure in MeCP2 mutant mice and the effects of IGF-1 treatment. (A) Mean brain weight of P60 mice that were wild-type (WT), MeCP2 mutant (KO), or mutant treated with (1–3)IGF-1 (KO-T). MeCP2 mutant mice had reduced brain weight (WT, 0.41 ± 0.03 g, n = 10 mice; KO, 0.33 ± 0.02 g, n = 8 mice; P < 0.0001, two-tailed t test), and brain weight was elevated following treatment with (1–3)IGF-1 from approximately P15 onward (KO-T, 0.36 ± 0.02 g, n = 10 mice; KO vs. KO-T P < 0.05, two-tailed t test). ***: P < 0.0001; *: P < 0.05. (B) Immunostaining in motor cortex layer 5 for the synaptic scaffolding protein PSD-95 in wild-type mice (WT), untreated mutant mice (KO), and mutant mice treated with (1–3)IGF-1 (KO-T). (Scale bar: 25 μm.) (C) Quantitation of PSD-95 immunostaining depicting average labeling intensity normalized to wild-type intensity levels. Mutant animals (KO) exhibited significantly reduced levels of PSD-95 compared to wild-type (WT) (relative KO/WT expression level, 0.43 ± 0.11; P < 0.01, two-tailed t test, comparing WT and KO levels). Treatment with (1–3)IGF-1 (KO-T) increased PSD-95 levels significantly (KO-T/WT expression level, 0.70 ± 0.02; P < 0.05, two-tailed t test, comparing KO and KO-T levels). **: P < 0.01; *: P < 0.05. (D) Golgi staining of layer 5 pyramidal cells in adult motor cortex to enable specific, sparse labeling of neurons and spine morphology. (Scale bar: 25 μm.) (E) Imaging at higher magnification (100×) to enable clear identification of dendritic spines. (Scale bar: 1.25 μm.) (F) Spine density in adult (P60) animals is reduced in MeCP2 mutant mice and reversed by (1–3)IGF-1 treatment from P15 onward (WT, 1.98 ± 0.14 spines/μm, n = 110 spines, 6 cells, 2 mice; KO, 1.05 ± 0.22 spines/μm, n = 133 spines, 5 cells, 3 mice; KO-T, 1.68 ± 0.14 spines/μm, n = 142 spines, 7 cells, 3 mice; P < 0.05, two-tailed t test, comparing WT vs. KO and KO vs. KO-T). *: P < 0.05.
Fig. 3.
Changes in synaptic transmission in MeCP2 mutant mice and the effects of IGF-1 treatment. (A) Representative traces from intracellular recordings of spontaneous excitatory postsynaptic currents (EPSCs) in acute slices from P28–32 sensorimotor cortex. Traces are presented for wild type (WT), mutant (KO), or mutant treated with (1–3)IGF-1 from P13–15 onward (KO-T). (B) Distributions of observed EPSC amplitudes measured across multiple cells, indicating a significant decrease in the size of EPSCs in mutant animals (WT, n = 1543 events, 11 cells; KO, 717 events, 6 cells, P < 0.00001 Kolmogorov-Smirnov test). Treatment with (1–3)IGF-1 partially but significantly reversed this trend (KO-T, n = 1723 events, 7 cells; KO-T vs. KO P < 0.00001 Kolmogorov-Smirnov test). (C) Mean EPSC amplitude, as in (B), for cells in each group. EPSCs are smaller in KO animals but larger with treatment of (1–3)IGF-1 (WT 17.3 ± 2.9 pA, KO 6.8 ± 0.8 pA, KO-T 9.1 ± 0.6 pA; WT vs. KO P < 0.01, KO vs. KO-T P < 0.05, two-tailed t test_)._ **: P < 0.01; *: P < 0.05.
Fig. 4.
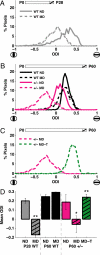
Changes in cortical plasticity in MeCP2 mutant mice and the effects of IGF-1 treatment. (A) Ocular dominance index (ODI) scores observed for all pixels in visual cortex of two representative young (P28) wild-type animals, one of which was nondeprived (ND) and the other monocularly deprived (MD). Schematic at top depicts the 4 day period within the animal's lifespan at which deprivation occurred in the MD animal (hatched). Compared to the normal control (“WT ND,” n = 1071 pixels), a population ODI shift was observed in the MD animal in favor of the open eye (“WT MD,” n = 1026 pixels). (D) shows the comparison of ODI values across animals. (B) ODI scores from representative adult (P60) animals that were nondeprived (ND) or monocularly deprived (MD) for 4 days. In adult wild-type mice (black lines), a significant population ODI shift was not observed when comparing the monocularly deprived animal (“WT MD,” n = 1092 pixels) to its nondeprived counterpart (“WT ND,” n = 2746 pixels). In contrast, adult MeCP2+/− female mice (pink lines) did undergo a shift in population ODI following monocular deprivation (“+/- MD,” n = 2670 pixels), compared to nondeprived mutants (“+/- ND,” n = 1484 pixels) indicating that these adult mice exhibit similar cortical plasticity to the young mice depicted in A. (D) shows the comparison of ODI values across animals. (C) Pink line: adult MeCP2+/− female mouse that had undergone the MD-induced plasticity in ODI (“+/- MD”; same animal as in B). Green line: representative adult MeCP2+/− mouse that had been monocularly deprived for 4 days and treated with (1–3)IGF-1 (“+/- MD-T,” n = 3074 pixels) from the first day of deprivation onward. Here the ODI of the treated mouse was significantly reversed from the mutant mouse, indicating that cortical plasticity had been abolished, preserving an ocular dominance profile typical of adult animals (as in B, “WT ND” or “WT MD”). (D) shows the comparison of ODI values across animals. (D) Mean ODI values for developing wild-type mice (P28 WT, left), adult wild-type mice (P60 WT, middle), and adult MeCP2 deficient mice (P60 +/-, right). Positive ODI values indicate higher drive from the contralateral eye, and thus preserved organization, while reduced or negative values indicate higher drive from the ipsilateral eye, and thus altered circuitry. In young wild-type animals (gray bars, left), MD leads to a significant overall shift in ODI (“ND” 0.20 ± 0.02, n = 3 animals; “MD” −0.17 ± 0.10, n = 4 animals; P < 0.01, two-tailed t test). In adult wild-type animals (black bars, middle), MD does not lead to a significant shift in ODI (“ND” 0.25 ± 0.02, n = 5 animals; “MD” 0.29 ± 0.04, n = 5 animals). In adult MeCP2 deficient animals (pink bars, right), MD does shift ODI values significantly (“ND” 0.18 ± 0.08, n = 5 animals; “MD” −0.06 ± 0.09, n = 5 animals; P < 0.05, two-tailed t test), as in the left gray bars. However, treatment with (1–3)IGF-1 from the first day of deprivation onward (green bar, right) prevents the shift in ODI (“MD-T” 0.24 ± 0.03, n = 5 animals; P < 0.01, two-tailed t test), such that responses are similar to wild-type adult animals. The MeCP2 deficient group only included female mice; the wild-type groups included both male and female mice since their ODI values were similar. **: P < 0.01; *: P < 0.05.
Similar articles
- Functional recovery with recombinant human IGF1 treatment in a mouse model of Rett Syndrome.
Castro J, Garcia RI, Kwok S, Banerjee A, Petravicz J, Woodson J, Mellios N, Tropea D, Sur M. Castro J, et al. Proc Natl Acad Sci U S A. 2014 Jul 8;111(27):9941-6. doi: 10.1073/pnas.1311685111. Epub 2014 Jun 23. Proc Natl Acad Sci U S A. 2014. PMID: 24958891 Free PMC article. - Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations.
Chapleau CA, Calfa GD, Lane MC, Albertson AJ, Larimore JL, Kudo S, Armstrong DL, Percy AK, Pozzo-Miller L. Chapleau CA, et al. Neurobiol Dis. 2009 Aug;35(2):219-33. doi: 10.1016/j.nbd.2009.05.001. Epub 2009 May 12. Neurobiol Dis. 2009. PMID: 19442733 Free PMC article. - Jointly reduced inhibition and excitation underlies circuit-wide changes in cortical processing in Rett syndrome.
Banerjee A, Rikhye RV, Breton-Provencher V, Tang X, Li C, Li K, Runyan CA, Fu Z, Jaenisch R, Sur M. Banerjee A, et al. Proc Natl Acad Sci U S A. 2016 Nov 15;113(46):E7287-E7296. doi: 10.1073/pnas.1615330113. Epub 2016 Nov 1. Proc Natl Acad Sci U S A. 2016. PMID: 27803317 Free PMC article. - Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome.
Filosa S, Pecorelli A, D'Esposito M, Valacchi G, Hajek J. Filosa S, et al. Free Radic Biol Med. 2015 Nov;88(Pt A):81-90. doi: 10.1016/j.freeradbiomed.2015.04.019. Epub 2015 May 8. Free Radic Biol Med. 2015. PMID: 25960047 Review. - Rett syndrome: from bed to bench.
Weng SM, Bailey ME, Cobb SR. Weng SM, et al. Pediatr Neonatol. 2011 Dec;52(6):309-16. doi: 10.1016/j.pedneo.2011.08.002. Epub 2011 Nov 6. Pediatr Neonatol. 2011. PMID: 22192257 Review.
Cited by
- Insulin-like growth factor-1 rescues synaptic and motor deficits in a mouse model of autism and developmental delay.
Bozdagi O, Tavassoli T, Buxbaum JD. Bozdagi O, et al. Mol Autism. 2013 Apr 27;4(1):9. doi: 10.1186/2040-2392-4-9. Mol Autism. 2013. PMID: 23621888 Free PMC article. - The Pathophysiology of Rett Syndrome With a Focus on Breathing Dysfunctions.
Ramirez JM, Karlen-Amarante M, Wang JJ, Bush NE, Carroll MS, Weese-Mayer DE, Huff A. Ramirez JM, et al. Physiology (Bethesda). 2020 Nov 1;35(6):375-390. doi: 10.1152/physiol.00008.2020. Physiology (Bethesda). 2020. PMID: 33052774 Free PMC article. Review. - Progress toward treatments for synaptic defects in autism.
Delorme R, Ey E, Toro R, Leboyer M, Gillberg C, Bourgeron T. Delorme R, et al. Nat Med. 2013 Jun;19(6):685-94. doi: 10.1038/nm.3193. Epub 2013 Jun 6. Nat Med. 2013. PMID: 23744158 Review. - Rett Syndrome: Reaching for Clinical Trials.
Pozzo-Miller L, Pati S, Percy AK. Pozzo-Miller L, et al. Neurotherapeutics. 2015 Jul;12(3):631-40. doi: 10.1007/s13311-015-0353-y. Neurotherapeutics. 2015. PMID: 25861995 Free PMC article. Review. - MeCP2 mutation results in compartment-specific reductions in dendritic branching and spine density in layer 5 motor cortical neurons of YFP-H mice.
Stuss DP, Boyd JD, Levin DB, Delaney KR. Stuss DP, et al. PLoS One. 2012;7(3):e31896. doi: 10.1371/journal.pone.0031896. Epub 2012 Mar 7. PLoS One. 2012. PMID: 22412847 Free PMC article.
References
- Chahrour M, Zoghbi HY. The story of rett syndrome: From clinic to neurobiology. Neuron. 2007;56:422–437. - PubMed
- Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
- Nan X, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. - PubMed
- Cohen DR, et al. Expression of MeCP2 in olfactory receptor neurons is developmentally regulated and occurs before synaptogenesis. Mol Cell Neurosci. 2003;22:417–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-CA087869/CA/NCI NIH HHS/United States
- R01-HD045022/HD/NICHD NIH HHS/United States
- F32 EY017500/EY/NEI NIH HHS/United States
- F32-EYO17240/PHS HHS/United States
- R01 HD045022/HD/NICHD NIH HHS/United States
- R01 CA087869/CA/NCI NIH HHS/United States
- F32-EY017500/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous