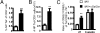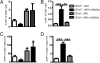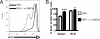Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity - PubMed (original) (raw)
Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity
Carole Guillonneau et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Current influenza A virus vaccines do not generate significant immunity against serologically distinct influenza A virus subtypes and would thus be ineffective in the face of a pandemic caused by a novel variant emerging from, say, a wildlife reservoir. One possible solution would be to modify these vaccines so that they prime cross-reactive CD8(+) cytotoxic T lymphocytes (CTL) cell-mediated immunity directed at conserved viral epitopes. A further strategy is to use novel adjuvants, such as the immunomodulatory glycolipid alpha-galactosylceramide (alpha-GalCer). We show here that giving alpha-GalCer with an inactivated influenza A virus has the paradoxical effect of diminishing acute CTL immunity via natural killer T (NKT) cell-dependent expression of indoleamine 2,3-dioxygenase (IDO), an important mediator of immune suppression, while at the same time promoting the survival of long-lived memory CTL populations capable of boosting protection against heterologous influenza A virus challenge. This enhancement of memory was likely due to the alpha-GalCer-induced upregulation of prosurvival genes, such as bcl-2, and points to the potential of alpha-GalCer as an adjuvant for promoting optimal, vaccine-induced CD8(+) T cell memory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
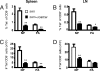
Fig. 1.
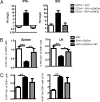
Quantitative analysis of DbNP366+ and DbPA224+ CD8+ T cells following influenza vaccination with an adjuvant. The B6 mice were immunized with iIAV ± α-GalCer, and lymphocytes from the spleen and brachial lymph node (BLN) were sampled 7 days later. The percentage (A and B) and absolute number (C and D) of tetramer-positive populations was evaluated among the CD8+ T cells in the spleen (A and C) and BLN (B and D). Results are expressed as mean ± SD for groups of 5 mice. *P < 0.05; **P < 0.01.
Fig. 2.
α-GalCer promotes the generation of long-term CD8+ T cell memory. The B6 mice were immunized with iIAV ± α-GalCer and killed 6 weeks later. Splenocytes were harvested and analyzed for the percentage (A) and absolute number (B) of tetramer-positive CD8+ CTLs. (C) The proportion of TCM vs. TEM cells was evaluated at early and late time points after immunization. Results are expressed as mean ± SD for groups of 5 mice. *P ≤ 0.05; **P < 0.01
Fig. 3.
NKT cells limit the acute CTL response but are essential for memory. Groups of CD1d+/+ and CD1d−/− mice were immunized with iIAV ± α-GalCer and analyzed 7 days (A and C) or 6 weeks later (B and D) for the percentage (A and B) and absolute number (C and D) of DbNP366+CD8+ T cells in the spleen. Results are expressed as mean ± SD. *P ≤ 0.05; **P < 0.01.
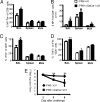
Fig. 4.
Increased protection of mice vaccinated with α-GalCer after infection. Mice were challenged i.n. with 104 pfu of live HKx31 IAV at 6 weeks after priming with iIAV + (black bars) or − (white bars) α-GalCer. The percentage (A) and absolute numbers (B) of influenza-specific CTLs were evaluated for the bronchoalveolar lavage (BAL), spleen, and mediastinal lymph node (MLN). Cells were stained for IFNγ and TNFα after stimulation with NP366 peptide. Shown is the percentage of IFNγ+ CD8+ T cells (C) and the proportion of IFNγ+ T cells that are also TNFα+ (D). *P ≤ 0.05 and **P < 0.01 comparing iIAV ± α-GalCer. (E) Virus clearance from the lung of mice that had been primed with iIAV ± α-GalCer. The graphs show the mean pfu per lung (n = 5) ± SD. **P < 0.01.
Fig. 5.
Increased expression of IFNγ and IDO inhibit T cell proliferation. (A) Quantitative analysis of IFNγ and IDO transcript accumulation was performed for the LNs of CD1d+/+ and CD1d−/− mice given iIAV ± α-GalCer. Results are expressed in arbitrary units (log2) of molecules normalized to hypoxanthine phosphoribosyltransferase ± SD for groups of 9 to 3 mice. (B) Mice were vaccinated with iIAV ± α-GalCer, and some were given 1-MT for 7 days. The proportion of CTLs was evaluated in the spleen (Left) and the LN (Right). Results are expressed as mean ± SD for groups of 10 mice. (C) Mice were analyzed 6 weeks after treatment for the percentage and number of CTLs in the spleen. Results are expressed in mean ± SD for groups of 5 mice. **P ≤ 0.001.
Fig. 6.
Enhanced memory-cell generation correlates with increased survival signals. (A) Representative histograms of intracellular Bcl-2 expression by splenic CD8+CD44+ T cells from mice given iIAV + (Right) or − (Left) a-GalCer. (B) Quantitation of the proportion of CD8+CD44+ CTLs expressing intracellular Bcl2 at 7 days after iIAV ± α-GalCer. Results are expressed as mean ± SD for groups of 5 mice. *P ≤ 0.05; ***P < 0.001.
Similar articles
- Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.
Zhang H, Zheng H, Guo P, Hu L, Wang Z, Wang J, Ju Y, Meng S. Zhang H, et al. J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article. - Mechanism of NKT cell activation by intranasal coadministration of alpha-galactosylceramide, which can induce cross-protection against influenza viruses.
Kamijuku H, Nagata Y, Jiang X, Ichinohe T, Tashiro T, Mori K, Taniguchi M, Hase K, Ohno H, Shimaoka T, Yonehara S, Odagiri T, Tashiro M, Sata T, Hasegawa H, Seino KI. Kamijuku H, et al. Mucosal Immunol. 2008 May;1(3):208-18. doi: 10.1038/mi.2008.2. Epub 2008 Mar 5. Mucosal Immunol. 2008. PMID: 19079180 - Activated iNKT cells promote memory CD8+ T cell differentiation during viral infection.
Reilly EC, Thompson EA, Aspeslagh S, Wands JR, Elewaut D, Brossay L. Reilly EC, et al. PLoS One. 2012;7(5):e37991. doi: 10.1371/journal.pone.0037991. Epub 2012 May 23. PLoS One. 2012. PMID: 22649570 Free PMC article. - Recalling the Future: Immunological Memory Toward Unpredictable Influenza Viruses.
Auladell M, Jia X, Hensen L, Chua B, Fox A, Nguyen THO, Doherty PC, Kedzierska K. Auladell M, et al. Front Immunol. 2019 Jul 2;10:1400. doi: 10.3389/fimmu.2019.01400. eCollection 2019. Front Immunol. 2019. PMID: 31312199 Free PMC article. Review. - Prospects for an influenza vaccine that induces cross-protective cytotoxic T lymphocytes.
Brown LE, Kelso A. Brown LE, et al. Immunol Cell Biol. 2009 May-Jun;87(4):300-8. doi: 10.1038/icb.2009.16. Epub 2009 Mar 24. Immunol Cell Biol. 2009. PMID: 19308073 Review.
Cited by
- LFA-1 Ligation by High-Density ICAM-1 Is Sufficient To Activate IFN-γ Release by Innate T Lymphocytes.
Sharma A, Lawry SM, Klein BS, Wang X, Sherer NM, Zumwalde NA, Gumperz JE. Sharma A, et al. J Immunol. 2018 Oct 15;201(8):2452-2461. doi: 10.4049/jimmunol.1800537. Epub 2018 Aug 31. J Immunol. 2018. PMID: 30171164 Free PMC article. - Investigation of CD4 and CD8 T cell-mediated protection against influenza A virus in a cohort study.
Tsang TK, Lam KT, Liu Y, Fang VJ, Mu X, Leung NHL, Peiris JSM, Leung GM, Cowling BJ, Tu W. Tsang TK, et al. BMC Med. 2022 Jul 21;20(1):230. doi: 10.1186/s12916-022-02429-7. BMC Med. 2022. PMID: 35858844 Free PMC article. - Universal immunity to influenza must outwit immune evasion.
Quiñones-Parra S, Loh L, Brown LE, Kedzierska K, Valkenburg SA. Quiñones-Parra S, et al. Front Microbiol. 2014 Jun 12;5:285. doi: 10.3389/fmicb.2014.00285. eCollection 2014. Front Microbiol. 2014. PMID: 24971078 Free PMC article. Review. - The burgeoning family of unconventional T cells.
Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. Godfrey DI, et al. Nat Immunol. 2015 Nov;16(11):1114-23. doi: 10.1038/ni.3298. Nat Immunol. 2015. PMID: 26482978 Review. - Harnessing Invariant NKT Cells to Improve Influenza Vaccines: A Pig Perspective.
Yang G, Richt JA, Driver JP. Yang G, et al. Int J Mol Sci. 2017 Dec 27;19(1):68. doi: 10.3390/ijms19010068. Int J Mol Sci. 2017. PMID: 29280974 Free PMC article. Review.
References
- de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol. 2000;61:94–99. - PubMed
- Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302:1519–1522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 081569/2/06/2/WT_/Wellcome Trust/United Kingdom
- AI70251/AI/NIAID NIH HHS/United States
- G0400421/MRC_/Medical Research Council/United Kingdom
- G0500590/MRC_/Medical Research Council/United Kingdom
- R01 AI070251/AI/NIAID NIH HHS/United States
- G9901077/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials