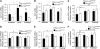Iron is essential for neuron development and memory function in mouse hippocampus - PubMed (original) (raw)
Iron is essential for neuron development and memory function in mouse hippocampus
Erik S Carlson et al. J Nutr. 2009 Apr.
Abstract
Iron deficiency (ID) is the most prevalent micronutrient deficiency in the world and it affects neurobehavioral outcome. It is unclear whether the effect of dietary ID on the brain is due to the lack of neuronal iron or from other processes occurring in conjunction with ID (e.g. hypoxia due to anemia). We delineated the role of murine Slc11a2 [divalent metal ion transporter-1 (DMT-1)] in hippocampal neuronal iron uptake during development and memory formation. Camk2a gene promoter-driven cre recombinase (Cre) transgene (Camk2a-Cre) mice were mated with Slc11a2 flox/flox mice to obtain nonanemic Slc11a2(hipp/hipp) (double mutant, hippocampal neuron-specific knockout of Slc11a2(hipp/hipp)) mice, the first conditionally targeted model of iron uptake in the brain. Slc11a2(hipp/hipp) mice had lower hippocampal iron content; altered developmental expression of genes involved in iron homeostasis, energy metabolism, and dendrite morphogenesis; reductions in markers for energy metabolism and glutamatergic neurotransmission on magnetic resonance spectroscopy; and altered pyramidal neuron dendrite morphology in area 1 of Ammon's Horn in the hippocampus. Slc11a2(hipp/hipp) mice did not reach the criterion on a difficult spatial navigation test but were able to learn a spatial navigation task on an easier version of the Morris water maze (MWM). Learning of the visual cued task did not differ between the Slc11a2(WT/WT) and Slc11a2(hipp/hipp) mice. Slc11a2(WT/WT) mice had upregulation of genes involved in iron uptake and metabolism in response to MWM training, and Slc11a2(hipp/hipp) mice had differential expression of these genes compared with Slc11a2(WT/WT) mice. Neuronal iron uptake by DMT-1 is essential for normal hippocampal neuronal development and Slc11a2 expression is induced by spatial memory training. Deletion of Slc11a2 disrupts hippocampal neuronal development and spatial memory behavior.
Figures
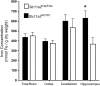
FIGURE 1
Regional iron concentrations in brains of _Slc11a2_WT/WT and _Slc11a2_hipp/hipp mice. Values are means ± SEM [_Slc11a2_WT/WT: n = 9 total brains, 14 cortices, 13 cerebellum pools (4 cerebella/pool), 6 hippocampus pools (14 hippocampi/pool); _Slc11a2_hipp/hipp: n = 4 total brains, 11 cortices, 7 cerebellum pools (4 cerebella/pool), 4 hippocampus pools (8 hippocampi/pool)]. *Different from _Slc11a2_hipp/hipp, P < 0.05.
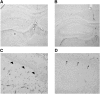
FIGURE 2
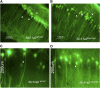
Perl's staining in hippocampus at P25 in _Slc11a2_WT/WT (n = 4) (A,C) and _Slc11a2_hipp/hipp (B,D) (n = 4) mice. Photomicrographs are at 100× (A,B) and 200× (C,D). Hippocampal area CA1 pyramidal neuronal soma in _Slc11a2_hipp/hipp mice show less staining (arrows) than _Slc11a2_WT/WT mice (arrowheads), whereas nonpyramidal cells localized between the hippocampal CA subfields and dentate gyrus retain positivity.
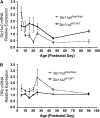
FIGURE 3
Hippocampal gene expression of Slc11a2 exon 7 (A) and TfRc (B) in _Slc11a2_WT/WT and _Slc11a2_hipp/hipp mice from P5 to P90. Values are means ± SEM, n = 3–5. Significant effects (P < 0.05): A, Cre status, Age, Cre status × Age ; B, Cre status, Age.
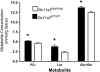
FIGURE 4
Hippocampus metabolite concentrations of _Slc11a2_hipp/hipp and _Slc11a2_WT/WT mice at P90. Values are means ± SEM, n = 6. *Different from _Slc11a2_hipp/hipp, P < 0.05. Lac, lactate; PCr, phosphocreatine.
FIGURE 5
Visualization of pyramidal apical dendrite main shaft lengths in area CA1 of hippocampus of P45 _Slc11a2_WT/WT (n = 9) (A,C) and _Slc11a2_hipp/hipp (n = 4) (B,D) mice. Cell bodies are oriented longitudinally across the upper right of each panel, directionally denoted by the arrow. Apical dendrites in _Slc11a2_WT/WT mice and _Slc11a2_hipp/hipp mice are marked with arrowheads. Magnification at × 200 (A,B) and × 400 (C,D).
FIGURE 6
Spatial navigation memory in 3-mo-old _Slc11a2_hipp/hipp (n = 15) and _Slc11a2_WT/WT (n = 12) mice in the standard MWM (version 1). Values are means ± SEM. (A) Percent of total swim time spent in target quadrant on probe trials after each day of training. Significant effects (P < 0.05): Cre status, Training, Cre status × Training. (B) Percent time spent floating during probe trials. Significant effects (P < 0.05): Cre status, Training. (C) Mean escape latencies by trial across 3 training days. Significant effects (P < 0.05): Cre status, Training. (D) Percent time spent in target quadrant by trial across 3 training days. Significant effects (P < 0.05): Cre status, Training, Cre status × Training. (E) Mean swim velocity by trial across 3 training days. Significant effects (P < 0.05): Cre status, Training, Cre status × Training
FIGURE 7
Gene expression in untrained, trained on hard MWM version 1, and trained on easier MWM version 2 _Slc11a2_WT/WT and _Slc11a2_hipp/hipp mice on P 90, quantified by qPCR. In each panel, the Y-axis represents units of relative mRNA expression with a value of 1.0 set for untrained _Slc11a2_WT/WT mice. Values are means ± SEM, n = 7–10 or 4 (untrained). Asterisks indicate that the designated groups differ: *P < 0.05; **P < 0.01 (unpaired 2-tailed Student's t tests). Significant effects in the 2-way ANOVA were: (A) Slc11a2; Cre status, MWM version, Cre status × MWM version; (B) Tfrc; Cre status, MWM version; (C) Aco1; MWM version; (D) Ireb2; Cre status, MWM version; (E) Grin2b; Cre status × MWM version; (F) Camk2a; MWM version, Cre status × MWM version.
Similar articles
- Hippocampus specific iron deficiency alters competition and cooperation between developing memory systems.
Carlson ES, Fretham SJ, Unger E, O'Connor M, Petryk A, Schallert T, Rao R, Tkac I, Georgieff MK. Carlson ES, et al. J Neurodev Disord. 2010 Sep;2(3):133-43. doi: 10.1007/s11689-010-9049-0. Epub 2010 May 9. J Neurodev Disord. 2010. PMID: 20824191 Free PMC article. - Iron Deficiency Impairs Developing Hippocampal Neuron Gene Expression, Energy Metabolism, and Dendrite Complexity.
Bastian TW, von Hohenberg WC, Mickelson DJ, Lanier LM, Georgieff MK. Bastian TW, et al. Dev Neurosci. 2016;38(4):264-276. doi: 10.1159/000448514. Epub 2016 Sep 27. Dev Neurosci. 2016. PMID: 27669335 Free PMC article. - Eltrombopag, a thrombopoietin mimetic, crosses the blood-brain barrier and impairs iron-dependent hippocampal neuron dendrite development.
Bastian TW, Duck KA, Michalopoulos GC, Chen MJ, Liu ZJ, Connor JR, Lanier LM, Sola-Visner MC, Georgieff MK. Bastian TW, et al. J Thromb Haemost. 2017 Mar;15(3):565-574. doi: 10.1111/jth.13602. Epub 2017 Feb 16. J Thromb Haemost. 2017. PMID: 28005311 Free PMC article. - The role of iron in learning and memory.
Fretham SJ, Carlson ES, Georgieff MK. Fretham SJ, et al. Adv Nutr. 2011 Mar;2(2):112-21. doi: 10.3945/an.110.000190. Epub 2011 Mar 10. Adv Nutr. 2011. PMID: 22332040 Free PMC article. Review. - Spatial Navigation (Water Maze) Tasks.
Terry AV Jr. Terry AV Jr. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 13. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 13. PMID: 21204326 Free Books & Documents. Review.
Cited by
- Knockdown of microglial iron import gene, Slc11a2, worsens cognitive function and alters microglial transcriptional landscape in a sex-specific manner in the APP/PS1 model of Alzheimer's disease.
Robertson KV, Rodriguez AS, Cartailler JP, Shrestha S, Schleh MW, Schroeder KR, Valenti AM, Kramer AT, Harrison FE, Hasty AH. Robertson KV, et al. J Neuroinflammation. 2024 Sep 27;21(1):238. doi: 10.1186/s12974-024-03238-w. J Neuroinflammation. 2024. PMID: 39334471 Free PMC article. - Knockdown of microglial iron import gene, DMT1, worsens cognitive function and alters microglial transcriptional landscape in a sex-specific manner in the APP/PS1 model of Alzheimer's disease.
Robertson KV, Rodriguez AS, Cartailler JP, Shrestha S, Schroeder KR, Valenti AM, Harrison FE, Hasty AH. Robertson KV, et al. Res Sq [Preprint]. 2024 Jun 27:rs.3.rs-4559940. doi: 10.21203/rs.3.rs-4559940/v1. Res Sq. 2024. PMID: 38978579 Free PMC article. Updated. Preprint. - Impact of Maternal Environment and Inflammation on Fetal Neurodevelopment.
Lubrano C, Parisi F, Cetin I. Lubrano C, et al. Antioxidants (Basel). 2024 Apr 11;13(4):453. doi: 10.3390/antiox13040453. Antioxidants (Basel). 2024. PMID: 38671901 Free PMC article. Review. - Biomarkers of Brain Dysfunction in Perinatal Iron Deficiency.
Rao RB. Rao RB. Nutrients. 2024 Apr 8;16(7):1092. doi: 10.3390/nu16071092. Nutrients. 2024. PMID: 38613125 Free PMC article. Review. - Research progress on ferroptosis in gliomas (Review).
Bo Y, Mu L, Yang Z, Li W, Jin M. Bo Y, et al. Oncol Lett. 2023 Nov 27;27(1):36. doi: 10.3892/ol.2023.14169. eCollection 2024 Jan. Oncol Lett. 2023. PMID: 38108075 Free PMC article. Review.
References
- Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–65. - PubMed
- Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier RA. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55:1034–41. - PubMed
- Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr. 1996;126:693–701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD 29421/HD/NICHD NIH HHS/United States
- R21 HD054490/HD/NICHD NIH HHS/United States
- R01 HL51057/HL/NHLBI NIH HHS/United States
- F31-NS047876/NS/NINDS NIH HHS/United States
- R01 DK053813-10/DK/NIDDK NIH HHS/United States
- R01 DK53813/DK/NIDDK NIH HHS/United States
- R01 DK053813/DK/NIDDK NIH HHS/United States
- F31 NS047876/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases