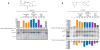The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator - PubMed (original) (raw)
The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator
Dominique Fontanilla et al. Science. 2009.
Abstract
The sigma-1 receptor is widely distributed in the central nervous system and periphery. Originally mischaracterized as an opioid receptor, the sigma-1 receptor binds a vast number of synthetic compounds but does not bind opioid peptides; it is currently considered an orphan receptor. The sigma-1 receptor pharmacophore includes an alkylamine core, also found in the endogenous compound N,N-dimethyltryptamine (DMT). DMT acts as a hallucinogen, but its receptor target has been unclear. DMT bound to sigma-1 receptors and inhibited voltage-gated sodium ion (Na+) channels in both native cardiac myocytes and heterologous cells that express sigma-1 receptors. DMT induced hypermobility in wild-type mice but not in sigma-1 receptor knockout mice. These biochemical, physiological, and behavioral experiments indicate that DMT is an endogenous agonist for the sigma-1 receptor.
Figures
Fig. 1
Sigma-1 receptor ligand pharmacophore and binding affinities. (A) A basic sigma-1 receptor ligand pharmacophore variant of Glennon et al. (6) was derived by removal of the red bonds from the sigma-1 receptor ligands fenpropimorph, haloperidol, and cocaine. (B) Competitive binding curves of tryptamine,_N_-methyltryptamine, and DMT, against the radioactive sigma-1 receptor ligand [3H]-(+)-pentazocine. Curves are shown as percent specific binding (5 μM haloperidol). (C) Sigma-1 and sigma-2 receptor _K_d values of trace amines and their _N_-methylated and N,_N_-dimethylated derivatives (scheme S2). Included are SEM values (n = 3 binding experiments) and _R_2 values for a nonlinear regression curve fit. Solid arrows denote the direction of increasing affinity.
Fig. 2
Tryptamine, _N_-methyltryptamine, and DMT inhibition of photolabeling. Rat liver membranes (100 μg per lane) were suspended in the presence or absence of the protecting drugs. Samples were photolyzed with (A) 1 nM carrier-free [125I]-IACoc or (B) 1 nM carrier-free [125I]IAF. Ten micromolar (+)-pentazocine (P) protected sigma-1 receptor photolabeling, whereas 10 μM haloperidol (H) protected both sigma-1 and sigma-2 receptors. Percent band intensities are shown as compared to controls performed in the absence of protecting ligand (−).
Fig. 3
Sodium channel inhibition by DMT. (A) In the presence or absence of 10 μM haloperidol, wild type (WT) orsigma-1receptorknock-out (KO) mouse liver homogenates (200 μg/lane) were photolabeled with 1 nM [125I]IAF. (B) Examples of _I_Na evoked by steps from −80 to −10 mV in HEK293 or COS-7 cells expressing hNav1.5 channel in the absence (control, black), presence (DMT, red), and after wash out (recovery, blue) of 100 μM DMT. Average inhibition by DMT was determined by measuring peak _I_Na. Bars represent mean ± SEM (n = 3 cells). _I_Na inhibition in HEK293 cells differed significantly from that in COS-7 cells (*P < 0.03). (C) Examples of _I_Na evoked as described in (B) in neonatal cardiac myocytes from WT and KO mice in the absence (control, black), presence (DMT, red), and after wash out (recovery, blue) of 100 μM DMT. Current inhibition in WT was significantly different from that in KO (*P < 0.002, n = 7 neonatal cardiac myocytes).
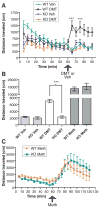
Fig. 4
DMT-induced hypermobility abrogated in the sigma-1 KO mouse. (A) Distances traveled by WT and KO mice were measured in an open-field assay in 5-min increments. Pargyline was injected 2 hours before DMT or vehicle (Veh) ip injection. Bars represent mean ± SEM (n = 8 to 14 mice). WT mice showed a significant (***P < 0.0001) increase in mobility in response to DMT as compared to KO mice. (B) Total distance traveled over 30 min after DMT, vehicle (Veh), or methamphetamine (Meth, n = 6 mice) injection in WT and KO mice. (C) Methamphetamine serves as a positive control for hypermobility in KO mice.
Similar articles
- When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor.
Su TP, Hayashi T, Vaupel DB. Su TP, et al. Sci Signal. 2009 Mar 10;2(61):pe12. doi: 10.1126/scisignal.261pe12. Sci Signal. 2009. PMID: 19278957 Free PMC article. Review. - Antagonist action of progesterone at σ-receptors in the modulation of voltage-gated sodium channels.
Johannessen M, Fontanilla D, Mavlyutov T, Ruoho AE, Jackson MB. Johannessen M, et al. Am J Physiol Cell Physiol. 2011 Feb;300(2):C328-37. doi: 10.1152/ajpcell.00383.2010. Epub 2010 Nov 17. Am J Physiol Cell Physiol. 2011. PMID: 21084640 Free PMC article. - Voltage-gated sodium channel modulation by sigma-receptors in cardiac myocytes and heterologous systems.
Johannessen M, Ramachandran S, Riemer L, Ramos-Serrano A, Ruoho AE, Jackson MB. Johannessen M, et al. Am J Physiol Cell Physiol. 2009 May;296(5):C1049-57. doi: 10.1152/ajpcell.00431.2008. Epub 2009 Mar 11. Am J Physiol Cell Physiol. 2009. PMID: 19279232 Free PMC article. - Psychedelic N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells.
Szabo A, Kovacs A, Frecska E, Rajnavolgyi E. Szabo A, et al. PLoS One. 2014 Aug 29;9(8):e106533. doi: 10.1371/journal.pone.0106533. eCollection 2014. PLoS One. 2014. PMID: 25171370 Free PMC article. - Significance of mammalian N, N-dimethyltryptamine (DMT): A 60-year-old debate.
Jiménez JH, Bouso JC. Jiménez JH, et al. J Psychopharmacol. 2022 Aug;36(8):905-919. doi: 10.1177/02698811221104054. Epub 2022 Jun 13. J Psychopharmacol. 2022. PMID: 35695604 Review.
Cited by
- Why N,N-dimethyltryptamine matters: unique features and therapeutic potential beyond classical psychedelics.
Chaves C, Dos Santos RG, Dursun SM, Tusconi M, Carta MG, Brietzke E, Hallak JEC. Chaves C, et al. Front Psychiatry. 2024 Nov 6;15:1485337. doi: 10.3389/fpsyt.2024.1485337. eCollection 2024. Front Psychiatry. 2024. PMID: 39568756 Free PMC article. - Sigma-1 receptor signaling: A potential therapeutic approach for ischemic stroke.
Ngo A, Fattakhov N, Toborek M. Ngo A, et al. J Cereb Blood Flow Metab. 2024 Dec;44(12):1430-1440. doi: 10.1177/0271678X241281547. Epub 2024 Sep 9. J Cereb Blood Flow Metab. 2024. PMID: 39246093 Free PMC article. Review. - Epigenetic mechanisms of rapid-acting antidepressants.
Inserra A, Campanale A, Rezai T, Romualdi P, Rubino T. Inserra A, et al. Transl Psychiatry. 2024 Sep 4;14(1):359. doi: 10.1038/s41398-024-03055-y. Transl Psychiatry. 2024. PMID: 39231927 Free PMC article. Review. - Serotonergic psychedelic 5-MeO-DMT alters plasticity-related gene expression and generates anxiolytic effects in stressed mice.
Nogueira M, Ferreira Golbert DC, Menezes R, Nóbrega de Almeida R, Galvão-Coelho NL, Siroky AN, Lima TZ, Maia H, Leão KE, Leão RN. Nogueira M, et al. Mol Psychiatry. 2025 Jan;30(1):50-60. doi: 10.1038/s41380-024-02655-w. Epub 2024 Jul 5. Mol Psychiatry. 2025. PMID: 38969716 - Insight into binding of endogenous neurosteroid ligands to the sigma-1 receptor.
Fu C, Xiao Y, Zhou X, Sun Z. Fu C, et al. Nat Commun. 2024 Jul 4;15(1):5619. doi: 10.1038/s41467-024-49894-7. Nat Commun. 2024. PMID: 38965213 Free PMC article.
References
- Hayashi T, Su TP. CNS Drugs. 2004;18:269. - PubMed
- Bouchard P, et al. Eur J Neurosci. 1995;7:1952. - PubMed
- Su TP, Weissman AD, Yeh SY. Life Sci. 1986;38:2199. - PubMed
- Su TP, London ED, Jaffe JH. Science. 1988;240:219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM008688/GM/NIGMS NIH HHS/United States
- F31 DA022932/DA/NIDA NIH HHS/United States
- T32 GM08688/GM/NIGMS NIH HHS/United States
- R01 NS030016-09/NS/NINDS NIH HHS/United States
- NS30016/NS/NINDS NIH HHS/United States
- R37 NS030016/NS/NINDS NIH HHS/United States
- R01 NS030016-08/NS/NINDS NIH HHS/United States
- R01 NS030016/NS/NINDS NIH HHS/United States
- R01 MH065503-01A1/MH/NIMH NIH HHS/United States
- R01 MH065503/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases