Amyloid-beta protofibril levels correlate with spatial learning in Arctic Alzheimer's disease transgenic mice - PubMed (original) (raw)
Amyloid-beta protofibril levels correlate with spatial learning in Arctic Alzheimer's disease transgenic mice
Anna Lord et al. FEBS J. 2009 Feb.
Abstract
Oligomeric assemblies of amyloid-beta (Abeta) are suggested to be central in the pathogenesis of Alzheimer's disease because levels of soluble Abeta correlate much better with the extent of cognitive dysfunctions than do senile plaque counts. Moreover, such Abeta species have been shown to be neurotoxic, to interfere with learned behavior and to inhibit the maintenance of hippocampal long-term potentiation. The tg-ArcSwe model (i.e. transgenic mice with the Arctic and Swedish Alzheimer mutations) expresses elevated levels of Abeta protofibrils in the brain, making tg-ArcSwe a highly suitable model for investigating the pathogenic role of these Abeta assemblies. In the present study, we estimated Abeta protofibril levels in the brain and cerebrospinal fluid of tg-ArcSwe mice, and also assessed their role with respect to cognitive functions. Protofibril levels, specifically measured with a sandwich ELISA, were found to be elevated in young tg-ArcSwe mice compared to several transgenic models lacking the Arctic mutation. In aged tg-ArcSwe mice with considerable plaque deposition, Abeta protofibrils were approximately 50% higher than in younger mice, whereas levels of total Abeta were exponentially increased. Young tg-ArcSwe mice showed deficits in spatial learning, and individual performances in the Morris water maze were correlated inversely with levels of Abeta protofibrils, but not with total Abeta levels. We conclude that Abeta protofibrils accumulate in an age-dependent manner in tg-ArcSwe mice, although to a far lesser extent than total Abeta. Our findings suggest that increased levels of Abeta protofibrils could result in spatial learning impairment.
Figures
Figure 1. Age-dependent changes in Aβ protofibril levels and total Aβ levels in tg-ArcSwe mice
(A) Levels of protofibrils in nontransgenic (non-tg) and tg-ArcSwe mice at 2 (n=6), 4 (n=9), 10 (n=11), 14 (n=10) and 17 (n=6) months (mo) of age. Aβ protofibrils remained rather stable with age but increased approximately 50% from 10 to 14 months of age. (B) Total Aβ levels in the same set of mice increased dramatically after plaque onset, here represented by age groups of 10 months and older. (C) Aβ protofibrils as a fraction out of total Aβ (in %) was highest in young (4 mo) tg-ArcSwe mice and markedly decreased with age. (** P<0.01 and *** P<0.001, by one-way ANOVA and Tukey’s multiple comparison post hoc test).
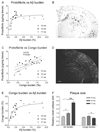
Figure 2. Aβ protofibril levels in tg-ArcSwe mice and their relation to plaque pathology
(A) Increased immunohistochemical Aβ burden was accompanied by raised Aβ protofibril levels in 10 (n=7) and 14 (n=10) months (mo) old animal. With a further increase in Aβ burden, at 17 mo (n=6), protofibril concentrations remained relatively stable. (C) When protofibril levels were compared to the extent of Congo red positive deposition, there was a significant correlation with linear regression when all animals were analyzed as a single group. (B, D) Representative pictures of immunohistochemical staining of Aβ burden and Congo red positive deposits converted to grayscale. The scale bars measure 200 µm. (E) Increased Congo red burden was paralleled by elevated Aβ burden at early stages of plaque accumulation (10 and 14 mo) but at the stage of advanced amyloid pathology (17 mo), the Congo red burden remained stable. (F) Relative mean plaque size of age groups 10, 14 and 17 mo was investigated. Plaque size at 10 months of age was set to 1. The average size of Aβ deposits increased drastically with age whereas the size of Congo red positive material remained relatively stable. (* P<0.05 and *** P<0.001, by one-way ANOVA and Tukey’s multiple comparison post hoc test).
Figure 3. Aβ protofibril levels in different APP transgenic models
Aβ protofibrils in TBS-soluble cortical extracts from several APP transgenic mouse lines were measured with mAb158 protofibril ELISA. (A) Aβ protofibril levels were elevated in 2 months old tg2576 (n=7) and PSAPP (n=5) mice as compared to nontransgenic (non-tg) littermates (n=4), but were significantly lower than in age-matched tg-ArcSwe (n=6) mice. Aβ protofibril levels did not differ between young tg2576 and PSAPP mice. (B) Aβ protofibril levels were increased by more than 3-fold in cortical extracts (ctx) of 22–27 months old tg2576 (n=8) and PSAPP (n=5) mice. Cerebellar extracts (cer) from the same set of transgenic mice were essentially devoid of Aβ protofibrils, although a few tg2576 mice had measurable levels in the cerebellum. (** P<0.01 and *** P<0.001, by one-way ANOVA and Tukey’s multiple comparison post hoc test).
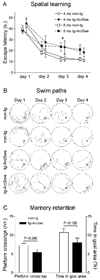
Figure 4. Spatial learning and memory in tg-ArcSwe mice
Transgenic (tg-ArcSwe) and nontransgenic (non-tg) mice were tested in the Morris water maze at 4 months (4 mo) and 8 months (8 mo) of age, n=17 (non-tg) and 20 (tg-ArcSwe) in total. (A) Escape latency in seconds (s.) was used as a measure of spatial learning. Each point in the figure represents average performance at each day ± standard error of the mean. Different age groups (4 and 8 mo) were offset in the figure for the sake of clarity. Learning was modestly impaired in tg-ArcSwe mice as compared to non-tg littermates with a significant effect of both genotype and time in a two-way factorial ANOVA. Fischer LSD pos-hoc showed longer escape latencies of tg-ArcSwe mice at day 3 (* P<0.05). There was no evidence that age affected performance in an initial single variance analysis (P=0.74). (B) Representative swim paths of two non-tg mice (upper panels) and two tg-ArcSwe mice (lower panels). Arrowheads (▷) illustrate the start position of each mouse. (C) In the probe trial, 72 h after last training session, non-tg mice crossed the platform more often than tg-ArcSwe mice and also spent more time in the goal area, but these differences did not reach significance.
Figure 5. Aβ levels in tg-ArcSwe mice without plaque pathology and their relation to spatial learning
Aβ protofibril levels in TBS-extracts and total Aβ levels in formic acid extracted brains of 4 months old tg-ArcSwe mice were investigated and related to spatial learning (n=9). (A) Aβ protofibrils inversely correlated with improvement in escape latency, measured as performance in the last trial subtracted from performance at the first acquisition session. (B) Levels of total Aβ, in the same set of mice, were not associated with improved escape latency and spatial learning.
Similar articles
- Antibody-Based In Vivo PET Imaging Detects Amyloid-β Reduction in Alzheimer Transgenic Mice After BACE-1 Inhibition.
Meier SR, Syvänen S, Hultqvist G, Fang XT, Roshanbin S, Lannfelt L, Neumann U, Sehlin D. Meier SR, et al. J Nucl Med. 2018 Dec;59(12):1885-1891. doi: 10.2967/jnumed.118.213140. Epub 2018 May 31. J Nucl Med. 2018. PMID: 29853653 Free PMC article. - The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice.
Tucker S, Möller C, Tegerstedt K, Lord A, Laudon H, Sjödahl J, Söderberg L, Spens E, Sahlin C, Waara ER, Satlin A, Gellerfors P, Osswald G, Lannfelt L. Tucker S, et al. J Alzheimers Dis. 2015;43(2):575-88. doi: 10.3233/JAD-140741. J Alzheimers Dis. 2015. PMID: 25096615 - An amyloid-beta protofibril-selective antibody prevents amyloid formation in a mouse model of Alzheimer's disease.
Lord A, Gumucio A, Englund H, Sehlin D, Sundquist VS, Söderberg L, Möller C, Gellerfors P, Lannfelt L, Pettersson FE, Nilsson LN. Lord A, et al. Neurobiol Dis. 2009 Dec;36(3):425-34. doi: 10.1016/j.nbd.2009.08.007. Epub 2009 Aug 22. Neurobiol Dis. 2009. PMID: 19703562 - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review. - Protofibrils of Amyloid-β are Important Targets of a Disease-Modifying Approach for Alzheimer's Disease.
Ono K, Tsuji M. Ono K, et al. Int J Mol Sci. 2020 Jan 31;21(3):952. doi: 10.3390/ijms21030952. Int J Mol Sci. 2020. PMID: 32023927 Free PMC article. Review.
Cited by
- The Arctic/Swedish APP mutation alters the impact of chronic stress on cognition in mice.
Cortese A, Delgado-Morales R, Almeida OFX, Romberg C. Cortese A, et al. Eur J Neurosci. 2019 Sep;50(5):2773-2785. doi: 10.1111/ejn.14500. Epub 2019 Jul 13. Eur J Neurosci. 2019. PMID: 31231836 Free PMC article. - Antibody-Based In Vivo PET Imaging Detects Amyloid-β Reduction in Alzheimer Transgenic Mice After BACE-1 Inhibition.
Meier SR, Syvänen S, Hultqvist G, Fang XT, Roshanbin S, Lannfelt L, Neumann U, Sehlin D. Meier SR, et al. J Nucl Med. 2018 Dec;59(12):1885-1891. doi: 10.2967/jnumed.118.213140. Epub 2018 May 31. J Nucl Med. 2018. PMID: 29853653 Free PMC article. - A light at the end of the tunnel - from mutation identification to a potential treatment for Alzheimer's disease.
Lannfelt L. Lannfelt L. Ups J Med Sci. 2023 Nov 28;128. doi: 10.48101/ujms.v128.10316. eCollection 2023. Ups J Med Sci. 2023. PMID: 38084203 Free PMC article. Review. - The Alzheimer's disease 5xFAD mouse model is best suited to investigate pretargeted imaging approaches beyond the blood-brain barrier.
Lopes van den Broek S, Sehlin D, Andersen JV, Aldana BI, Beschörner N, Nedergaard M, Knudsen GM, Syvänen S, Herth MM. Lopes van den Broek S, et al. Front Nucl Med. 2022 Sep 23;2:1001722. doi: 10.3389/fnume.2022.1001722. eCollection 2022. Front Nucl Med. 2022. PMID: 39390994 Free PMC article. - Blood-brain barrier penetrating neprilysin degrades monomeric amyloid-beta in a mouse model of Alzheimer's disease.
Rofo F, Metzendorf NG, Saubi C, Suominen L, Godec A, Sehlin D, Syvänen S, Hultqvist G. Rofo F, et al. Alzheimers Res Ther. 2022 Dec 5;14(1):180. doi: 10.1186/s13195-022-01132-2. Alzheimers Res Ther. 2022. PMID: 36471433 Free PMC article.
References
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. - PubMed
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. - PubMed
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–866. - PubMed
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG 18478/AG/NIA NIH HHS/United States
- AG 25509/AG/NIA NIH HHS/United States
- AG15490/AG/NIA NIH HHS/United States
- AG 25711/AG/NIA NIH HHS/United States
- R01 AG025509/AG/NIA NIH HHS/United States
- R01 AG018478/AG/NIA NIH HHS/United States
- AG04418/AG/NIA NIH HHS/United States
- P01 AG004418/AG/NIA NIH HHS/United States
- P50 AG025711/AG/NIA NIH HHS/United States
- R01 AG015490/AG/NIA NIH HHS/United States
- R01 AG015490-09A1/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous