Cell fate regulation by coupling mechanical cycles to biochemical signaling pathways - PubMed (original) (raw)
Review
Cell fate regulation by coupling mechanical cycles to biochemical signaling pathways
Viola Vogel et al. Curr Opin Cell Biol. 2009 Feb.
Abstract
Many aspects of cellular motility and mechanics are cyclic in nature such as the extension and retraction of lamellipodia or filopodia. Inherent to the cycles of extension and retraction that test the environment is the production of mechano-chemical signals that can alter long-term cell behavior, transcription patterns, and cell fate. We are just starting to define such cycles in several aspects of cell motility, including periodic contractions, integrin cycles of binding and release as well as the normal oscillations in motile activity. Cycles of local cell contraction and release are directly coupled to cycles of stressing and releasing extracellular contacts (matrix or cells) as well as cytoplasmic mechanotransducers. Stretching can alter external physical properties or sites exposed by matrix molecules as well as internal networks; thus, cell contractions can cause a secondary wave of mechano-regulated outside-in and internal cell signal changes. In some cases, the integration of both external and internal signals in space and time can stimulate a change in cell state from quiescence to growth or differentiation. In this review we will develop the basic concept of the mechano-chemical cycles and the ways in which they can be described and understood.
Figures
Figure 1
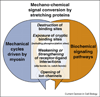
Mechanical and biochemical signaling networks are coupled through proteins that serve as mechano-chemical signal converters. Protein stretching can cause a multitude of functional changes (as recently reviewed in [6••,12,1•]). Force-induced alterations of the equilibrium structure of proteins can destroy molecular binding motifs, expose cryptic binding sites that are buried in native protein folds ([13,14•,15•,16•,81••,82] and recently reviewed in [17]). This includes exposing phosphorylation sites [18••], force furthermore either accelerates the dissociation of non-covalent bonds or activates catch bonds that bind more tightly when activated by force [19,20••,26••,28,43]. Finally, membrane stretching can lead to the opening of ion channels [,–23].
Figure 2
Force-bearing protein network that links the cytoskeleton to the extracellular matrix. The molecular motor myosin (red) pulls on an actin fiber thereby applying force to a protein network (green) that physically links the cytoskeleton to the extracellular matrix. Diverse proteins that are associated with the various molecules of the force-bearing network are given in yellow. Other force-bearing networks exist too, including those that link cytoskeletal elements to cell-cell junctions.
Figure 3
Micrographs of cells spreading on surfaces of different rigidities show different spreading patterns. (A) DIC images of the first and last point show the extent of spreading of a MEF on a stiff polyacrylamide. The kymograph of the same MEF spreading on a stiff polyacrylamide gel (20% acrylamide, 0.8% bisacrylamide) covalently linked with FN 10 µg/ml shows that periodic contractions were generated. (B) DIC images and kymograph of a MEF spreading on an intermediary stiffness polyacrylamide gel (10% acrylamide, 0.1% bisacrylamide) covalently linked with FN 10 µg/ml. Note the effective protrusion of the leading edge without the generation of periodic contractions and the following global ruffling of the lamella. (C) DIC images and kymograph of a MEF spreading on a soft polyacrylamide gel (10% acrylamide, 0.04% bisacrylamide) covalently linked with FN 10 µg/ml. Note the absence of both periodic contractions and effective protrusion of the leading edge. (Left. Scale bars are equal to 5 m. Right. time bars are equal to 30 s; scale bars are equal to 2 m. Arrows indicate the direction of protrusion.)
Figure 4
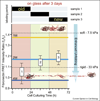
Fibronectin fibers are progressively more unfolded as the extracellular matrix ages as probed by fluorescence resonance energy transfer (FRET). Fibroblasts were seeded on glass and allowed to assemble matrix for three days in 10% serum. Trace amounts of FRET-labeled fibronectin were added for limited time periods as indicated in the upper bar graph. On average, each labeled fibronectin molecule carried seven donors and four acceptors. The FRET ratios probed in matrix (without optical cross-talk correction) are compared to those measured in solution in the presence of various concentrations of the denaturant GnHCl, where the onset of a loss of secondary structure is seen at 1 M GnHCl and beyond. Fibronectin is completely unfolded at 4 M GnHCl. After three days, the matrix deposited during the first 24 h is highly unfolded while the younger matrix is far less unfolded. Thus, the physical properties of matrix are changing as matrix ages (adopted from [77•]). For a comparison, the FRET ratios of fibronectin matrix assembled by fibroblasts on rigid and soft polyacrylamide surfaces are shown 4 h after cell seeding. As inserts, a fibronectin fragment of three type III modules is shown in a folded state as well as after partial unfolding by tensile mechanical force acting on its termini.
Similar articles
- The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells.
Mierke CT. Mierke CT. Rep Prog Phys. 2019 Jun;82(6):064602. doi: 10.1088/1361-6633/ab1628. Epub 2019 Apr 4. Rep Prog Phys. 2019. PMID: 30947151 Review. - Integrin and Its Associated Proteins as a Mediator for Mechano-Signal Transduction.
Katoh K. Katoh K. Biomolecules. 2025 Jan 23;15(2):166. doi: 10.3390/biom15020166. Biomolecules. 2025. PMID: 40001469 Free PMC article. Review. - Biochemistry and biomechanics of cell motility.
Li S, Guan JL, Chien S. Li S, et al. Annu Rev Biomed Eng. 2005;7:105-50. doi: 10.1146/annurev.bioeng.7.060804.100340. Annu Rev Biomed Eng. 2005. PMID: 16004568 Review. - Exploratory cell dynamics: a sense of touch for cells?
Nalbant P, Dehmelt L. Nalbant P, et al. Biol Chem. 2018 Jul 26;399(8):809-819. doi: 10.1515/hsz-2017-0341. Biol Chem. 2018. PMID: 29664730 Review. - Mechanosensing and mechanochemical transduction: how is mechanical energy sensed and converted into chemical energy in an extracellular matrix?
Silver FH, Siperko LM. Silver FH, et al. Crit Rev Biomed Eng. 2003;31(4):255-331. doi: 10.1615/critrevbiomedeng.v31.i4.10. Crit Rev Biomed Eng. 2003. PMID: 15095950 Review.
Cited by
- A molecular trajectory of α-actinin activation.
Shams H, Golji J, Mofrad MR. Shams H, et al. Biophys J. 2012 Nov 21;103(10):2050-9. doi: 10.1016/j.bpj.2012.08.044. Epub 2012 Nov 20. Biophys J. 2012. PMID: 23200039 Free PMC article. - New perspectives on the development of muscle contractures following central motor lesions.
Pingel J, Bartels EM, Nielsen JB. Pingel J, et al. J Physiol. 2017 Feb 15;595(4):1027-1038. doi: 10.1113/JP272767. Epub 2016 Dec 7. J Physiol. 2017. PMID: 27779750 Free PMC article. Review. - Dual modes of motility at the leading edge of migrating epithelial cell sheets.
Klarlund JK. Klarlund JK. Proc Natl Acad Sci U S A. 2012 Sep 25;109(39):15799-804. doi: 10.1073/pnas.1210992109. Epub 2012 Sep 10. Proc Natl Acad Sci U S A. 2012. PMID: 23019364 Free PMC article. - Dynamic regulation of the structure and functions of integrin adhesions.
Wolfenson H, Lavelin I, Geiger B. Wolfenson H, et al. Dev Cell. 2013 Mar 11;24(5):447-58. doi: 10.1016/j.devcel.2013.02.012. Dev Cell. 2013. PMID: 23484852 Free PMC article. Review. - Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions.
Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR. Albiges-Rizo C, et al. J Cell Sci. 2009 Sep 1;122(Pt 17):3037-49. doi: 10.1242/jcs.052704. J Cell Sci. 2009. PMID: 19692590 Free PMC article. Review.
References
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. This review details many of the mechanisms of cell mechanosensing and how response mechanisms must integrate the sensory signals
- Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Dobereiner HG, Freund Y, Borisy G, et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–575. This study characterizes the mechanical cycle of edge extension and retraction that is seen in many cells
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. Force, focal adhesion assembly a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. - PubMed
- Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. - PubMed
- Lele TP, Kumar S. Brushes cables anchors recent insights into multiscale assembly and mechanics of cellular structural networks. Cell Biochem Biophys. 2007;47:348–360. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources