FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling - PubMed (original) (raw)
FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling
BinQuan Zhuang et al. Neuron. 2009.
Abstract
The dendritic morphology of neurons dictates their abilities to process and transmit information; however, the signaling pathways that regulate dendritic growth and complexity are poorly understood. Here, we show that retinoids induce the expression of the FERM Rho-GEF protein FARP1 in the developing spinal cord. FARP1 is expressed in subsets of motor neurons and is enriched in dendrites of lateral motor column (LMC) neurons that innervate the limb. FARP1 is necessary and sufficient to promote LMC dendritic growth but does not affect dendrite number or axonal morphology. We show that FARP1 serves as a specific effector of transmembrane Semaphorin6A and PlexinA4 signals to regulate LMC dendritic growth, and that its Rho-GEF domain is necessary for this function. These findings reveal that retinoid and Sema6A/PlexA4 signaling pathways intersect through FARP1 to control dendritic growth, and uncover the existence of subtype-specific signaling networks that control dendritic developmental programs in spinal motor neurons.
Figures
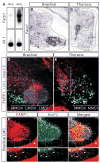
Figure 1. FARP1 is induced by RA and is localized to the dendrites of LMC motor neurons
(A) Reverse northern blot shows Farp1, but not control clone 29.1, is induced by addition of retinol (ROL). Two bands are seen in the ROL lane due to traces of plasmid containing FARP1 cDNA (lower band) in addition to the PCR-amplified FARP1 fragment (upper band). (B–E) Right hemisegments of transverse sections of St 31 chick spinal cord. (B, C) In situ hybridization of Farp1 mRNA. Hatched black lines outline the spinal cord. (D, E) Confocal micrographs detecting FARP1 protein (F–H′) Confocal micrographs of ventral right quadrants of St 31 chick spinal cords. Boxes in panels F–H are magnified in panels F′–H′. Arrowheads mark the dendrites with co-localization of FARP1 and MAP2 proteins. * marks FARP1 expression in condensing cartilage.
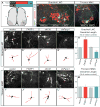
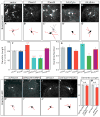
Figure 2. FARP1 regulates the growth of LMC neurites in vivo
(A) Schematic depicting strategy to visualize motor neuron dendrites in chick spinal cords. (B, C) Confocal micrographs of ventral right quadrants of St 31 spinal cords. Hatched white lines outline the spinal cord. (D–G; I–L) Confocal micrographs of representative brachial LMC (D–G) and thoracic MMC (I–L) motor neurons electroporated with either the vector, FARP1, short hairpin control (shCtrl), or short hairpin FARP1 (shFarp1) expressing constructs. (D′–G′; I′–L′) Traces of electroporated motor neurons, neurites traced in red were quantified; arrows mark traced neurons (see supplemental methods for details). Individual motor neuron subtypes were identified by costaining with specific molecular markers. (H) Graph of dendrite length of brachial LMC motor neurons; compared to the vector, FARP1 p=0.001, shCtrl p=0.32, shFarp1 p=7×10−15. (M) Graph of dendrite length of thoracic MMC motor neurons; compared to the vector, FARP1 p=0.99, shCtrl p=0.39, shFarp1 p=0.45. All graphs, mean ± s.e.m., two-tailed Student’s t-test, n= at least 50 motor neurons from 6 electroporated embryos.
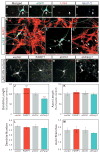
Figure 3. FARP1 regulates dendritic growth of LMC motor neurons in vitro
(A–E″) Confocal micrographs of cultured chick spinal neurons. White arrows in A–D mark the same neuron in panels of merged and split detection channels. Hatched box in E is magnified in panels E′ and E″. White asterisk marks the axon, white arrows mark dendrites. (F–I) Confocal micrographs of representative cultured LMC motor neurons electroporated with either vector, FARP1, shCtrl, or shFarp1. Red arrowheads mark the axons. (J) Graphs of dendrite length; compared to the vector, FARP1 p=0.0007, shCtrl p=0.28, shFarp1 p=6×10−7. (K) Graphs of axon length; compared to the vector, FARP1 p=0.48, shCtrl p=0.69, shFarp1 p=0.81. (L) Graphs of dendrite number; compared to the vector, FARP1 p=0.93, shCtrl p=0.85, shFarp1 p=0.76. (M) Graphs of axon branches, compared to the vector, FARP1 p=0.76, shCtrl p=0.97, shFarp1 p=0.29. All graphs, mean ± s.e.m; two-tailed Student’s t-test =dendrite and axon length, chi-square test =dendrite number and axon branch.
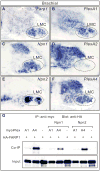
Figure 4. FARP1 preferentially interacts with PlexA4 independently of Npns
(A–F) In situ hybridization on transverse sections of St 31 right hemisegments of chick spinal cords. Hatched black lines outline LMC motor neurons. (G) Western blot analysis of co-IP experiments using HEK293T cells.
Figure 5. PlexA4 regulates dendritic growth of LMC motor neurons through FARP1
(A–E, H–K) Micrographs of representative electroporated brachial LMC motor neurons. (A′–E′; H′-K′) Traces of electroporated motor neurons, neurites traced in red were quantified; arrows mark traced neurons. (F) Graph depicting dendrite length of brachial LMC motor neurons; compared to the vector; PlexA1 p=0.75, PlexA4 p=4×10−5, shPlexA4 p=9×10−12, A4ΔCyto p=5×10−6, A4ΔEcto p=0.06.(G) Graph of dendrite number of brachial LMC motor neurons; compared to the vector; PlexA1 p=0.59, PlexA4 p=0.11, shPlexA4 p=0.53, A4ΔCyto p=0.01, A4ΔEcto p=0.52. (L, M) Graphs showing dendrite length of brachial LMC motor neurons; non-significant (N.S.) between shPlexA4 and _shPlexA4_+ FARP1, p= 0.64; N.S. between shFarp1 and shFARP1+PlexA4, p=0.27. All graphs, mean ± s.e.m., two-tailed Student’s t-test= dendrite length, chi-square test= dendrite number.
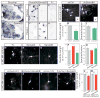
Figure 6. Sema6A signals through PlexA4 and FARP1 to regulate dendritic growth of LMC motor neurons
(A, B) In situ hybridization on transverse sections of right hemisegments of St 31 chick spinal cords. (C–H) Ligand-receptor binding experiments in COS−7 cells. (I, J) Micrographs of representative electroporated brachial LMC motor neurons. Boxed insets show traces of electroporated neurons marked by arrows. (K, L) Graphs of dendrite length (p=6×10−12) and number (p=0.44) of brachial LMC motor neurons. (M–O, R–U) Confocal micrographs of representative cultured LMC motor neurons treated with ligand combined with electroporation of shFarp1 or shPlexA4. Red arrowheads mark the axons. (P, V, W) Graphs of dendrite length of cultured LMC motor neurons; compared to the Fc, Sema6A p=0.004, Sema6D p=0.08, and shFarp1 N.S. p=0.15, shPlexA4 N.S. p=0.46. (Q) Graph of dendrite number of cultured LMC motor neurons; compared with Fc, Sema6A p=0.19, Sema6D p=0.34. For all graphs, mean ± s.e.m., two-tailed Student’s t-test = dendrite number, chi-square test = dendrite length.
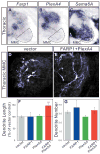
Figure 7. The FARP1-PlexA4 complex is sufficient to promote the dendritic growth of MMC motor neurons
(A–C) In situ hybridization on transverse sections of right hemisegments of St 31 chick spinal cords. Hatched black lines outline MMC motor neurons. (D, E) Confocal micrographs of representative electroporated thoracic MMC motor neurons. (F) Graphs of dendrite length of thoracic MMC motor neurons; compared to the vector, FARP1 p=0.99, PlexA4 p=0.76, FARP1+PlexA4 p=9×10−5. (G) Graphs of dendrite number of thoracic MMC motor neurons; compared to the vector, FARP1 p=0.29, PlexA4 p=0.16, FARP1+PlexA4 p=0.79. All graphs, mean ± s.e.m., two-tailed Student’s t-test = dendrite number, chi-square test = dendrite length.
Figure 8. FARP1 function requires the DH domain
(A) Western blot of co-IP experiments using HEK293T cells. (B, C) Confocal micrographs of representative LMC motor neurons electroporated with FARP1ΔDH cultured with Sema6A. Red arrowheads mark the axons. (D) Graph of dendrite length of cultured LMC motor neurons, N.S. p=0.62 (E–G) Confocal micrographs of representative electroporated brachial LMC motor neurons. (H) Graph of dendrite length of brachial LMC motor neurons; compared to the vector, FARP1ΔDH p=3×10−19, FARP1ΔDH+PlexA4 p=5×10−17, and N.S. p=0.41, all graphs, mean ± s.e.m., two-tailed Student’s t-test. (I) Model of FARP1 effector function. RA= retinoic acid. Although Sema6A and PlexA4 are shown acting in trans, it is possible that they function in cis.
Similar articles
- Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin-plexin mechanism.
Bron R, Vermeren M, Kokot N, Andrews W, Little GE, Mitchell KJ, Cohen J. Bron R, et al. Neural Dev. 2007 Oct 30;2:21. doi: 10.1186/1749-8104-2-21. Neural Dev. 2007. PMID: 17971221 Free PMC article. - Activity-dependent regulation of dendritic complexity by semaphorin 3A through Farp1.
Cheadle L, Biederer T. Cheadle L, et al. J Neurosci. 2014 Jun 4;34(23):7999-8009. doi: 10.1523/JNEUROSCI.3950-13.2014. J Neurosci. 2014. PMID: 24899721 Free PMC article. - The Rif GTPase regulates cytoskeletal signaling from plexinA4 to promote neurite retraction.
Fan L, Yan H, Pellegrin S, Morigen, Mellor H. Fan L, et al. Neurosci Lett. 2015 Mar 17;590:178-83. doi: 10.1016/j.neulet.2015.02.010. Epub 2015 Feb 7. Neurosci Lett. 2015. PMID: 25668492 - Retinoids run rampant: multiple roles during spinal cord and motor neuron development.
Appel B, Eisen JS. Appel B, et al. Neuron. 2003 Oct 30;40(3):461-4. doi: 10.1016/s0896-6273(03)00688-3. Neuron. 2003. PMID: 14642271 Review. - Regulators of Rho GTPases in neuronal development.
Watabe-Uchida M, Govek EE, Van Aelst L. Watabe-Uchida M, et al. J Neurosci. 2006 Oct 18;26(42):10633-5. doi: 10.1523/JNEUROSCI.4084-06.2006. J Neurosci. 2006. PMID: 17050702 Free PMC article. Review.
Cited by
- Structural basis for autoinhibition of the guanine nucleotide exchange factor FARP2.
He X, Kuo YC, Rosche TJ, Zhang X. He X, et al. Structure. 2013 Mar 5;21(3):355-64. doi: 10.1016/j.str.2013.01.001. Epub 2013 Jan 31. Structure. 2013. PMID: 23375260 Free PMC article. - Signaling from axon guidance receptors.
Bashaw GJ, Klein R. Bashaw GJ, et al. Cold Spring Harb Perspect Biol. 2010 May;2(5):a001941. doi: 10.1101/cshperspect.a001941. Epub 2010 Mar 24. Cold Spring Harb Perspect Biol. 2010. PMID: 20452961 Free PMC article. Review. - Membrane and Protein Interactions of the Pleckstrin Homology Domain Superfamily.
Lenoir M, Kufareva I, Abagyan R, Overduin M. Lenoir M, et al. Membranes (Basel). 2015 Oct 23;5(4):646-63. doi: 10.3390/membranes5040646. Membranes (Basel). 2015. PMID: 26512702 Free PMC article. - Mutation of Semaphorin-6A disrupts limbic and cortical connectivity and models neurodevelopmental psychopathology.
Rünker AE, O'Tuathaigh C, Dunleavy M, Morris DW, Little GE, Corvin AP, Gill M, Henshall DC, Waddington JL, Mitchell KJ. Rünker AE, et al. PLoS One. 2011;6(11):e26488. doi: 10.1371/journal.pone.0026488. Epub 2011 Nov 21. PLoS One. 2011. PMID: 22132072 Free PMC article. - FARP-1 deletion is associated with lack of response to autism treatment by early start denver model in a multiplex family.
Cucinotta F, Ricciardello A, Turriziani L, Calabrese G, Briguglio M, Boncoddo M, Bellomo F, Tomaiuolo P, Martines S, Bruschetta M, La Fauci Belponer F, Di Bella T, Colombi C, Baccarin M, Picinelli C, Castronovo P, Lintas C, Sacco R, Biederer T, Kellam B, Scherer SW, Persico AM. Cucinotta F, et al. Mol Genet Genomic Med. 2020 Sep;8(9):e1373. doi: 10.1002/mgg3.1373. Epub 2020 Jun 25. Mol Genet Genomic Med. 2020. PMID: 32588496 Free PMC article.
References
- Abu-Abed S, MacLean G, Fraulob V, Chambon P, Petkovich M, Dollé P. Differential expression of the retinoic acid-metabolizing enzymes CYP26A1 and CYP26B1 during murine organogenesis. Mech Dev. 2002;110:173–177. - PubMed
- Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–1304. - PubMed
- Cohen S, Funkelstein L, Livet J, Rougon G, Henderson CE, Castellani V, Mann F. A semaphorin code defines subpopulations of spinal motor neurons during mouse development. Euro J Neurosci. 2005;21:1767–76. - PubMed
- Corcoran J, So PL, Barber RD, Vencent KJ, Mazarakis ND, Mitrophanous KA, Kingsman SM, Maden M. Retinoic acid receptor beta2 and neurite outgrowth in the adult mouse spinal cord in vitro. J Cell Sci. 2002;115:3779–86. - PubMed
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS046336-02/NS/NINDS NIH HHS/United States
- R01 NS046336-01/NS/NINDS NIH HHS/United States
- R01 NS046336-04/NS/NINDS NIH HHS/United States
- R01 NS046336/NS/NINDS NIH HHS/United States
- R01 NS046336-03/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources