Role of the oestrogen receptors GPR30 and ERalpha in peripheral sensitization: relevance to trigeminal pain disorders in women - PubMed (original) (raw)
Role of the oestrogen receptors GPR30 and ERalpha in peripheral sensitization: relevance to trigeminal pain disorders in women
C S Liverman et al. Cephalalgia. 2009 Jul.
Abstract
Oestrogen increases facial allodynia through its actions on activation of the MAPK extracellular-signal regulated kinase (ERK) in trigeminal ganglion neurons. This goal of study was to determine which oestrogen receptor is required for behavioural sensitization. Immunohistochemical studies demonstrated the presence of oestrogen receptor alpha (ERalpha) in nuclei of larger neurons and cytoplasm of smaller neurons, and the novel oestrogen receptor G-protein coupled receptor 30 (GPR30) in small diameter neurons that also contained peripherin, a marker of unmyelinated C-fibres. Specific agonists for ERalpha (PPT) and GPR30 (G-1), but not ERbeta (DPN), activated ERK in trigeminal ganglion neurons in vitro. Both G-1 and PPT treatment increased allodynia after CFA injections into the masseter of ovariectomized Sprague-Dawley rats. Treatment with oestrogen increased expression of ERalpha but not GPR30, while masseter inflammation increased GRP30 but not ERalpha. Differential modulation of these ERK-coupled receptors by oestrogen and inflammation may play a role in painful episodes of temporomandibular disorder and migraine.
Figures
Figure 1
GPR30 protein is present in rat trigeminal ganglion. Western blot of trigeminal ganglion lysates from ovariectomized female rats labeled with antibodies to GPR30 (red) and the loading control GAPDH (green).
Figure 2
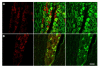
GPR30 is localized to unmyelinated neurons in the trigeminal ganglion A) Double-label immunohistochemistry for GPR30 and NFH, a marker of myelinated neurons. There is a small degree of overlap between GPR30 and NFH. B) Double-label immunohistochemistry for GPR30 and peripherin. GPR30 and peripherin are highly co-localized. Panels on the left are single-labeled for GPR30. The middle row of panels contains merged images of the left and right columns. The right row of panels are single-labeled neurofilament H (top) or peripherin (bottom). Scale bar (50 μm) applies to all images.
Figure 3
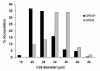
GPR30 is present in small diameter neurons. Histogram shows cell diameter measurements for trigeminal ganglion neurons that were GPR30 immunoreactive (black bars) and GPR30 non-immunoreactive (gray bars).
Figure 4
Cytoplasmic and nuclear ERERα immunoreactivity is present in the trigeminal ganglion. A) Immunohistochemistry for ERα in trigeminal ganglion from ovariectomized female rat. ERα staining was present in neurons in both cytoplasmic and nuclear locations B. Histogram showing cell diameter measurements for neurons that were immunoreactive for nuclear and cytoplasmic ERα. Cytoplasmic ERα was present in predominately small neurons and nuclear ERα was present in larger neurons.
Figure 5
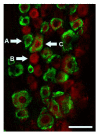
GPR30 and ERα are present in distinct but overlapping populations in the rat trigeminal ganglion. Immunohistochemistry for GPR30 (red) and ERα (green) shows ERα positive (A), GPR30 positive (B), and ERα /GPR30 double-positive (C) neurons. Scale bar (50 μm)
Figure 6
Selective activation of GPR30 or ERα activates ERK in trigeminal ganglion neurons in vitro. Cultured trigeminal ganglion neurons were treated with specific agonists for ERα (PPT, 10 nM), GPR30 (G-1, 1 μM), ERβ (DPN, 100 nM), 17β–estradiol (E2, 10nM), or vehicle. Following agonist treatment, cultures were stained with antibodies raised against the dually-phosphorylated form of ERK and the neuron-specific marker NeuN and the percentage of neurons immunoreactive for activated ERK was assessed. Data are presented as the mean percentage of NeuN positive neurons that were p-ERK positive from three independent experiments. (*=p<0.05 compared to vehicle, one-way ANOVA).
Figure 7
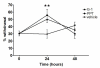
Selective activation of GPR30 or ERα enhances orofacial allodynia. Specific agonists for ERα (PPT) and GPR30 (G-1) increased withdrawal response to stimulation of the whisker pad in CFA-treated ovariectomized female rats. For each animal, the left masseter of was injected intra-muscularly with 50 μl CFA (1:1 in saline) and PPT (1 mg/kg), G-1 (1 mg/kg) or vehicle were injected subcutaneously. Withdrawal response to stimulation of the whisker pad with a von Frey filament (0.16g) was assessed at 24 hours after agonist administration. Data are shown as mean+/− SEM. N=8 per group; *=p<0.05 compared to vehicle, one-way ANOVA.
Figure 8
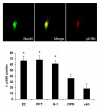
Estrogen treatment increases expression levels of ERα, but not GPR30, in the trigeminal ganglion of ovariectomized female rats. Estradiol valerate (10 μg/kg in sesame oil) or vehicle was administered by subcutaneous injection and trigeminal ganglia collected 24 hours later. A) Representative Western blots of trigeminal ganglion from ovariectomized rats treated with estrogen or vehicle. B) Effects of estrogen on protein level of the 66kD ERα isoform C) Effects of estrogen on the protein level of the 90kD ERα isoform D) Effects of estrogen on GPR30 protein levels. Data are shown as mean integrated intensity relative to GAPDH +/− SEM. (N=6 per group; *=p<0.05 compared to vehicle, t-test).
Figure 9
Peripheral inflammation increases expression of GPR30 but not ERα in trigeminal ganglion from ovariectomized female rats. CFA (1:1 in saline) or vehicle was administered intra-muscularly into the masseter muscle and trigeminal ganglia were collected 24 hours later. A) Representative Western blots of trigeminal ganglion from rats with or without masseter inflammation. B) Effects of inflammation of the masseter muscle on GPR30 protein level. C and D) Effects of inflammation of the masseter muscle on ERα protein level for the 66kD (C) and the 90 kD (D) isoforms. Data are shown as mean integrated intensity relative to GAPDH +/− SEM (N=6 per group; *=p<0.05 compared to vehicle, two-tailed t-test).
Similar articles
- Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia.
Liverman CS, Brown JW, Sandhir R, Klein RM, McCarson K, Berman NE. Liverman CS, et al. Cephalalgia. 2009 May;29(5):520-31. doi: 10.1111/j.1468-2982.2008.01755.x. Epub 2009 Feb 3. Cephalalgia. 2009. PMID: 19210515 Free PMC article. - Estradiol upregulates voltage-gated sodium channel 1.7 in trigeminal ganglion contributing to hyperalgesia of inflamed TMJ.
Bi RY, Meng Z, Zhang P, Wang XD, Ding Y, Gan YH. Bi RY, et al. PLoS One. 2017 Jun 5;12(6):e0178589. doi: 10.1371/journal.pone.0178589. eCollection 2017. PLoS One. 2017. PMID: 28582470 Free PMC article. - 17β-estradiol rapidly attenuates P2X3 receptor-mediated peripheral pain signal transduction via ERα and GPR30.
Lu Y, Jiang Q, Yu L, Lu ZY, Meng SP, Su D, Burnstock G, Ma B. Lu Y, et al. Endocrinology. 2013 Jul;154(7):2421-33. doi: 10.1210/en.2012-2119. Epub 2013 Apr 22. Endocrinology. 2013. PMID: 23610132 - Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats.
Bereiter DA, Cioffi JL, Bereiter DF. Bereiter DA, et al. Arch Oral Biol. 2005 Nov;50(11):971-9. doi: 10.1016/j.archoralbio.2005.03.010. Arch Oral Biol. 2005. PMID: 15893734 - Localization of estrogen receptor ERα, ERβ and GPR30 on myenteric neurons of the gastrointestinal tract and their role in motility.
Liu JYH, Lin G, Fang M, Rudd JA. Liu JYH, et al. Gen Comp Endocrinol. 2019 Feb 1;272:63-75. doi: 10.1016/j.ygcen.2018.11.016. Epub 2018 Nov 28. Gen Comp Endocrinol. 2019. PMID: 30502347
Cited by
- 17-β-Estradiol induces spreading depression and pain behavior in alert female rats.
Sandweiss AJ, Cottier KE, McIntosh MI, Dussor G, Davis TP, Vanderah TW, Largent-Milnes TM. Sandweiss AJ, et al. Oncotarget. 2017 Dec 9;8(69):114109-114122. doi: 10.18632/oncotarget.23141. eCollection 2017 Dec 26. Oncotarget. 2017. PMID: 29371973 Free PMC article. - Chronic 17β-estradiol pretreatment has pronociceptive effect on behavioral and morphological changes induced by orofacial formalin in ovariectomized rats.
Fejes-Szabó A, Spekker E, Tar L, Nagy-Grócz G, Bohár Z, Laborc KF, Vécsei L, Párdutz Á. Fejes-Szabó A, et al. J Pain Res. 2018 Sep 25;11:2011-2021. doi: 10.2147/JPR.S165969. eCollection 2018. J Pain Res. 2018. PMID: 30310305 Free PMC article. - Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER.
Prossnitz ER, Barton M. Prossnitz ER, et al. Prostaglandins Other Lipid Mediat. 2009 Sep;89(3-4):89-97. doi: 10.1016/j.prostaglandins.2009.05.001. Epub 2009 May 13. Prostaglandins Other Lipid Mediat. 2009. PMID: 19442754 Free PMC article. Review. - Behavioral effects and mechanisms of migraine pathogenesis following estradiol exposure in a multibehavioral model of migraine in rat.
Vermeer LM, Gregory E, Winter MK, McCarson KE, Berman NE. Vermeer LM, et al. Exp Neurol. 2015 Jan;263:8-16. doi: 10.1016/j.expneurol.2014.09.011. Epub 2014 Sep 28. Exp Neurol. 2015. PMID: 25263582 Free PMC article. - GPR30 disrupts the balance of GABAergic and glutamatergic transmission in the spinal cord driving to the development of bone cancer pain.
Luo J, Huang X, Li Y, Li Y, Xu X, Gao Y, Shi R, Yao W, Liu J, Ke C. Luo J, et al. Oncotarget. 2016 Nov 8;7(45):73462-73472. doi: 10.18632/oncotarget.11867. Oncotarget. 2016. PMID: 27608844 Free PMC article.
References
- LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8:291–305. - PubMed
- Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population--a prevalence study. J Clin Epidemiol. 1991;44:1147–1157. - PubMed
- Lipton RB, Stewart WF, Scher AI. Epidemiology and economic impact of migraine. Curr Med Res Opin. 2001;17(Suppl 1):s4–12. - PubMed
- Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. - PubMed
- Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355–365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous