Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3 epsilon upregulation - PubMed (original) (raw)
Comparative Study
. 2009 Mar 3;119(8):1124-34.
doi: 10.1161/CIRCULATIONAHA.108.812537. Epub 2009 Feb 16.
Wai-Mui Cheung, Yau-Sheng Tsai, Yi-Tong Chen, Wen-Hsuan Fong, Hsin-Da Tsai, Yu-Chang Chen, Jun-Yang Liou, Song-Kun Shyue, Jin-Jer Chen, Y Eugene Chen, Nobuyo Maeda, Kenneth K Wu, Teng-Nan Lin
Affiliations
- PMID: 19221220
- PMCID: PMC4144045
- DOI: 10.1161/CIRCULATIONAHA.108.812537
Comparative Study
Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3 epsilon upregulation
Jui-Sheng Wu et al. Circulation. 2009.
Abstract
Background: Thiazolidinediones have been reported to protect against ischemia-reperfusion injury. Their protective actions are considered to be peroxisome proliferator-activated receptor-gamma (PPAR-gamma)-dependent; however, it is unclear how PPAR-gamma activation confers resistance to ischemia-reperfusion injury.
Methods and results: We evaluated the effects of rosiglitazone or PPAR-gamma overexpression on cerebral infarction in a rat model and investigated the antiapoptotic actions in the N2-A neuroblastoma cell model. Rosiglitazone or PPAR-gamma overexpression significantly reduced infarct volume. The protective effect was abrogated by PPAR-gamma small interfering RNA. In mice with knock-in of a PPAR-gamma dominant-negative mutant, infarct volume was enhanced. Proteomic analysis revealed that brain 14-3-3epsilon was highly upregulated in rats treated with rosiglitazone. Upregulation of 14-3-3epsilon was abrogated by PPAR-gamma small interfering RNA or antagonist. Promoter analysis and chromatin immunoprecipitation revealed that rosiglitazone induced PPAR-gamma binding to specific regulatory elements on the 14-3-3epsilon promoter and thereby increased 14-3-3epsilon transcription. 14-3-3epsilon Small interfering RNA abrogated the antiapoptotic actions of rosiglitazone or PPAR-gamma overexpression, whereas 14-3-3epsilon recombinant proteins rescued brain tissues and N2-A cells from ischemia-induced damage and apoptosis. Elevated 14-3-3epsilon enhanced binding of phosphorylated Bad and protected mitochondrial membrane potential.
Conclusions: Ligand-activated PPAR-gamma confers resistance to neuronal apoptosis and cerebral infarction by driving 14-3-3epsilon transcription. 14-3-3epsilon Upregulation enhances sequestration of phosphorylated Bad and thereby suppresses apoptosis.
Figures
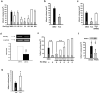
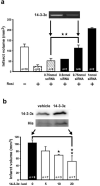
Figure 1. Rosiglitazone and PPAR-γ reduce ischemic brain injury in vivo
(a) Rosiglitazone was injected intraventricularly immediately after 30-min ischemia. Infarct volumes were determined after 24 h reperfusion. (b) Rosiglitazone (50 ng) was infused 2 h after a 30-min transient occlusion. (c) Rosiglitazone with & without GW9662 was infused. (d) PPAR-γ siRNA (0.5 nmol) or scRNA (2 nmol) was infused intraventricularly immediately after ischemia and PPAR-γ mRNA of brain tissues was measured 24 h later. The upper panel shows a representative gel and the lower panel, mean ± SD of densitometric analysis of four independent experiments. (e) Rosiglitazone with or without PPAR-γ siRNA was infused immediately after 30-min ischemia, and infarct volume was measured 24 h later. (f) Recombinant PPAR-γ protein (5 μg) was infused intraventricularly for 72 h before a 30-min ischemia. The inset indicates cortical PPAR-γ protein levels at 24 h after reperfusion. (g) PPAR-γ P465L dominant negative mutant mice (L/+) and wild type littermate controls (+/+) were subjected to 30-min ischemia and 24 h reperfusoin. Each bar denotes mean ± SD (n as indicated). *P<0.05. **P<0.01.
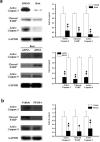
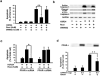
Figure 2. Analysis of apoptotic signals in ischemic brain
(a) Rosiglitazone (50 ng) or DMSO was injected with PPAR-γ siRNA or control scRNA, immediately after 30 min ischemia. Active caspases and cleaved PARP were analyzed by Western blotting. (b) Recombinant PPAR-γ proteins were infused for 72 h before I/R. Left panels show representative blots and right panels, densitometry of three experiments. *p<0.05; ** p<0.01.
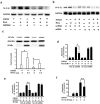
Figure 3. 14-3-3ε is increased in rosiglitazone-treated ischemic brain
(a) Rats were subjected to I/R with or without Rosi treatment. Proteins in rat brains were analyzed with 2-DGE. The insets show a spot with increased density in Rosi-treated vs. control brain. Analysis by LC-MS/MS identified this spot to be 14-3-3ε. Similar results were obtained in two other experiments. (b) Western blot analysis of 14-3-3ε in I/R vs. sham. Rats were treated with rosiglitazone or DMSO immediately after 30-min ischemia. Upper panel shows a representative blot and the lower panel the error bars from 3 independent experiments. Each bar denotes mean ± SD. *p<0.05, **p<0.01. (c) 14-3-3ε protein levels in brain tissues treated with or without rosiglitazone in the presence or absence of PPAR-γ siRNA or control scRNA. (d) 14-3-3ε proteins in brain tissues treated with recombinant PPAR-γ proteins or vehicle. (e) 14-3-3ε protein levels in wild-type (+/+) and L/+ mutant mouse brain tissues. Similar results were obtained in two other experiments.
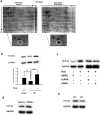
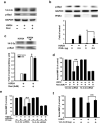
Figure 4. Ligand-activated PPAR-γ increases 14-3-3ε transcription
(a-d) 14-3-3ε proteins were analyzed by Western blotting in N2-A cells treated with rosiglitazone (a), rosiglitazone in the presence of GW9662 (b), rosiglitazone in the presence of PPAR-γ siRNA or scRNA (c), or PPAR-γ expression vectors (mPPAR-γ) (d). (e) & (f) N2-A cells transfected with 14-3-3ε promoter constructs p1625 or p1348 were treated with rosiglitazone (e) or PPAR-γ (f). Promoter activity was expressed as relative light unit (RLU) using β-gal (b-Gal) as control to normalize the activity. (g) ChIP analysis of PPAR-γ binding to the PPRE region (upper panel) of 14-3-3ε promoter. Binding of PPAR-β/δ was included as a control. Each bar represents mean ± SD of at least three independent experiments conducted in triplicate. *P<0.05. **P<0.01.
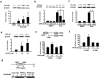
Figure 5. Control of cerebral infarction by 14-3-3ε in vivo
(a) Rosiglitazone with or without 14-3-3ε siRNA or scRNA was injected immediately after 30-min ischemia. Inset shows cortical 14-3-3ε mRNA levels. Similar results were obtained in two other experiments. (b) His-tagged 14-3-3ε recombinant proteins (5~20 μg) were infused 72 h before I/R. Inset shows 14-3-3ε and His analyzed by Western blotting. Each bar denotes mean ± SD. *P<0.05. **P<0.01. Similar results were obtained in two other experiments.
Figure 6. Rosiglitazone attenuates N2-A apoptosis in a PPAR-γ dependent manner
(a) Cells were subjected to OGD for 3h followed by reoxygenation for 24 h (H3R24) with or without rosiglitazone (Rosi) and/or GW9662. Apoptosis was analyzed by flow cytometry. (b) Cells were treated as a) and active caspase 3, 9 and cleaved PARP were determined by Western blotting. A representative blot is shown. (c) N2-A cells transfected with PPAR-γ siRNA or control were subjected to OGD (H3R24) with or without Rosi. (d) Cells transfected with PPAR-γ plasmids were subjected to H3R24. Upper panels show PPAR-γ proteins analyzed by Western blotting. Each bar denotes mean ± SD from at least three independent experiments conducted in triplicate. *P<0.05. **P<0.01.
Figure 7. Rosiglitazone and PPAR-γ restore 14-3-3ε
(a) N2-A cells were subjected to OGD (H3R24) in the presence or absence Rosi and GW9662, and protein levels of 14-3-3ε were measured. (b) Cells transfected with PPAR-γ siRNA or control scRNA were subjected to H3R24 in the presence or absence of Rosi. (c) Cells transfected with PPAR-γ plasmids were subjected to H3R24. The upper panel shows a representative blot and the lower panel, the densitometry analysis. (d) & (e) Cells transfected with 14-3-3ε siRNA or control scRNA were subjected to H3R24 with or without Rosi (d) or PPAR-γ transfection (e). (f) N2-A transfected with 14-3-3ε plasmids were subjected to H3R24. Each bar denotes mean ± SD of at least three independent experiments conducted in triplicate. *P<0.05. **P<0.01.
Figure 8. Interaction between 14-3-3ε and phosphorylated Bad (p-Bad)
(a) & (b) N2-A cells were subjected to H3R24 in the presence or absence of Rosi (a) or PPAR-γ (b). 14-3-3ε and p-Bad were analyzed by Western blotting. (c) Cells treated with H3R24 in the presence or absence of Rosi (R) were lysed and immunoprecipitated with a 14-3-3ε antibody. Proteins in the immunoprecipitate were analyzed by Western blotting using p-Bad or 14-3-3ε antibodies. Upper panels show representative immunoblots and the lower panels densitometry analysis. (d) & (e) Cells were transfected with 14-3-3ε siRNA or control and subjected to H3R12 in the presence or absence of Rosi (d) or PPAR-γ (e). MMP was analyzed by flow cytometry using JC-1 probe. (f) MMP was measured in cells treated with H3R12 in the presence or absence of 14-3-3ε plasmids. Each bar represents mean ± SD of at least three experiments. *P<0.05. **P<0.01.
Similar articles
- Anti-apoptotic actions of PPAR-gamma against ischemic stroke.
Fong WH, Tsai HD, Chen YC, Wu JS, Lin TN. Fong WH, et al. Mol Neurobiol. 2010 Jun;41(2-3):180-6. doi: 10.1007/s12035-010-8103-y. Epub 2010 Feb 3. Mol Neurobiol. 2010. PMID: 20127524 Review. - Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone.
Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Luo Y, et al. J Neurochem. 2006 Apr;97(2):435-48. doi: 10.1111/j.1471-4159.2006.03758.x. Epub 2006 Mar 15. J Neurochem. 2006. PMID: 16539667 - PPAR-γ Ameliorates Neuronal Apoptosis and Ischemic Brain Injury via Suppressing NF-κB-Driven p22phox Transcription.
Wu JS, Tsai HD, Cheung WM, Hsu CY, Lin TN. Wu JS, et al. Mol Neurobiol. 2016 Aug;53(6):3626-3645. doi: 10.1007/s12035-015-9294-z. Epub 2015 Jun 25. Mol Neurobiol. 2016. PMID: 26108185 - Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock.
Abdelrahman M, Sivarajah A, Thiemermann C. Abdelrahman M, et al. Cardiovasc Res. 2005 Mar 1;65(4):772-81. doi: 10.1016/j.cardiores.2004.12.008. Cardiovasc Res. 2005. PMID: 15721857 Review.
Cited by
- Ciglitazone, a novel inhibitor of lung apoptosis following hemorrhagic shock.
Chima RS, Hake PW, Piraino G, Mangeshkar P, O'Connor M, Zingarelli B. Chima RS, et al. Int J Clin Exp Med. 2010 Jan 1;3(1):1-9. Int J Clin Exp Med. 2010. PMID: 20369035 Free PMC article. - Anti-apoptotic actions of PPAR-gamma against ischemic stroke.
Fong WH, Tsai HD, Chen YC, Wu JS, Lin TN. Fong WH, et al. Mol Neurobiol. 2010 Jun;41(2-3):180-6. doi: 10.1007/s12035-010-8103-y. Epub 2010 Feb 3. Mol Neurobiol. 2010. PMID: 20127524 Review. - Protecting against vascular disease in brain.
Faraci FM. Faraci FM. Am J Physiol Heart Circ Physiol. 2011 May;300(5):H1566-82. doi: 10.1152/ajpheart.01310.2010. Epub 2011 Feb 18. Am J Physiol Heart Circ Physiol. 2011. PMID: 21335467 Free PMC article. Review. - The intrinsic PEDF is regulated by PPARγ in permanent focal cerebral ischemia of rat.
Zhu C, Zhang X, Qiao H, Wang L, Zhang X, Xing Y, Wang C, Dong L, Ji Y, Cao X. Zhu C, et al. Neurochem Res. 2012 Oct;37(10):2099-107. doi: 10.1007/s11064-012-0831-0. Epub 2012 Jun 20. Neurochem Res. 2012. PMID: 22714093 - Losartan protects liver against ischaemia/reperfusion injury through PPAR-γ activation and receptor for advanced glycation end-products down-regulation.
Koh EJ, Yoon SJ, Lee SM. Koh EJ, et al. Br J Pharmacol. 2013 Jul;169(6):1404-16. doi: 10.1111/bph.12229. Br J Pharmacol. 2013. PMID: 23647130 Free PMC article.
References
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-Δ12,14-Prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 1995;83:803–812. - PubMed
- Kliewer SA, Lenhard JM, Willson TM, Patel L, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–819. - PubMed
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor-γ (PPAR-γ). J. Biol. Chem. 1995;270:12953–12956. - PubMed
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK067320-04/DK/NIDDK NIH HHS/United States
- P50 NS023327-149001/NS/NINDS NIH HHS/United States
- R01 HL050675-07/HL/NHLBI NIH HHS/United States
- R01 HL089544-01A1/HL/NHLBI NIH HHS/United States
- P50 NS023327-17/NS/NINDS NIH HHS/United States
- R01 HL075397/HL/NHLBI NIH HHS/United States
- HL-75397/HL/NHLBI NIH HHS/United States
- P50 NS023327-169001/NS/NINDS NIH HHS/United States
- R01 HL068878-08S1/HL/NHLBI NIH HHS/United States
- P50 NS023327-150012/NS/NINDS NIH HHS/United States
- R01 HL068878-07S1/HL/NHLBI NIH HHS/United States
- P50 NS023327-139001/NS/NINDS NIH HHS/United States
- R01 HL068878-07/HL/NHLBI NIH HHS/United States
- R01 HL068878-01S2/HL/NHLBI NIH HHS/United States
- P50 NS023327-130007/NS/NINDS NIH HHS/United States
- P50 NS023327-14/NS/NINDS NIH HHS/United States
- R01 HL050675-08/HL/NHLBI NIH HHS/United States
- R37 HL042630/HL/NHLBI NIH HHS/United States
- R01 HL068878-05/HL/NHLBI NIH HHS/United States
- P50 NS023327-160011/NS/NINDS NIH HHS/United States
- R01 HL068878-02/HL/NHLBI NIH HHS/United States
- R01 HL068878/HL/NHLBI NIH HHS/United States
- P50 NS023327-15/NS/NINDS NIH HHS/United States
- P50 NS023327-129001/NS/NINDS NIH HHS/United States
- R01 HL075397-02/HL/NHLBI NIH HHS/United States
- R01 HL050675-12/HL/NHLBI NIH HHS/United States
- R01 HL068878-04/HL/NHLBI NIH HHS/United States
- R01 HL075397-03/HL/NHLBI NIH HHS/United States
- P50 NS023327-150011/NS/NINDS NIH HHS/United States
- HL-89544/HL/NHLBI NIH HHS/United States
- R01 HL050675-10/HL/NHLBI NIH HHS/United States
- HL-68878/HL/NHLBI NIH HHS/United States
- R01 HL075397-01A1S1/HL/NHLBI NIH HHS/United States
- R01 HL068878-08/HL/NHLBI NIH HHS/United States
- P50 NS023327-15S1/NS/NINDS NIH HHS/United States
- R01 HL089544-02/HL/NHLBI NIH HHS/United States
- R01 HL075397-01A1/HL/NHLBI NIH HHS/United States
- R01 HL089544/HL/NHLBI NIH HHS/United States
- P50 NS023327-160012/NS/NINDS NIH HHS/United States
- P50 NS023327-170012/NS/NINDS NIH HHS/United States
- P50 NS023327-18/NS/NINDS NIH HHS/United States
- HL-50675/HL/NHLBI NIH HHS/United States
- P50 NS023327-140011/NS/NINDS NIH HHS/United States
- P50 NS023327-179001/NS/NINDS NIH HHS/United States
- NS-23327/NS/NINDS NIH HHS/United States
- P50 NS023327/NS/NINDS NIH HHS/United States
- R01 HL050675-06/HL/NHLBI NIH HHS/United States
- R01 HL068878-03/HL/NHLBI NIH HHS/United States
- R01 HL075397-04/HL/NHLBI NIH HHS/United States
- R01 HL050675-11/HL/NHLBI NIH HHS/United States
- K02 HL105659/HL/NHLBI NIH HHS/United States
- R01 HL075397-05/HL/NHLBI NIH HHS/United States
- P50 NS023327-15S19001/NS/NINDS NIH HHS/United States
- P50 NS023327-170011/NS/NINDS NIH HHS/United States
- P50 NS023327-15S10012/NS/NINDS NIH HHS/United States
- P50 NS023327-120007/NS/NINDS NIH HHS/United States
- DK67320/DK/NIDDK NIH HHS/United States
- R01 HL050675-09/HL/NHLBI NIH HHS/United States
- R01 HL077145/HL/NHLBI NIH HHS/United States
- P50 NS023327-159001/NS/NINDS NIH HHS/United States
- R01 HL068878-09/HL/NHLBI NIH HHS/United States
- R01 HL068878-06/HL/NHLBI NIH HHS/United States
- R01 HL068878-05S1/HL/NHLBI NIH HHS/United States
- R01 HL050675/HL/NHLBI NIH HHS/United States
- P50 NS023327-140012/NS/NINDS NIH HHS/United States
- P50 NS023327-15S10011/NS/NINDS NIH HHS/United States
- R01 DK067320/DK/NIDDK NIH HHS/United States
- R01 HL068878-01/HL/NHLBI NIH HHS/United States
- R01 HL068878-01S1/HL/NHLBI NIH HHS/United States
- P50 NS023327-16/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials