Modularity and interactions in the genetics of gene expression - PubMed (original) (raw)
Modularity and interactions in the genetics of gene expression
Oren Litvin et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Understanding the effect of genetic sequence variation on phenotype is a major challenge that lies at the heart of genetics. We developed GOLPH (GenOmic Linkage to PHenotype), a statistical method to identify genetic interactions, and used it to characterize the landscape of genetic interactions between gene expression quantitative trait loci. Our results reveal that allele-specific interactions, in which a gene only exerts an influence on the phenotype in the presence of a particular allele at the primary locus, are widespread and that genetic interactions are predominantly nonadditive. The data portray a complex picture in which interacting loci influence the expression of modules of coexpressed genes involved in coherent biological processes and pathways. We show that genetic variation at a single gene can have a major impact on the global transcriptional response, altering interactions between genes through shutdown or activation of pathways. Thus, different cellular states occur not only in response to the external environment but also result from intrinsic genetic variation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Overview of the GOLPH algorithm. GOLPH takes as input gene expression and genotype data for a set of individuals. (Upper) The computation occurring at each stage. In stage 1 genes are linked to a primary locus; in stage 2 iQTL are constructed by partitioning the samples based on the primary locus and linkage to the secondary locus; and in stage 3, FDR is used to expand significant linkages. See
Figs. S1–S3
for more detail. (Lower) Once all iQTL modules have been constructed they are analyzed by using GENATOMY, our interactive visualization and data analysis tool. GENATOMY uses additional resources such as sequence, GO annotations, protein–DNA interactions, and genetic interactions to help interpret the data.
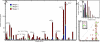
Fig. 2.
Stage 3 results in a marked increase in linked genes. The number of linked genes increases at each of the 3 stages, with the greatest expansion in module size occurring at stage 3. (A) Number of linkages at each locus color-coded by stage 1 (blue), 2 (green), and 3 (red). The x axis represents the location of the locus, each of the bold lines below the axis represents yeast chromosomes I–XVI. The y axis represents the number of genes linked to that locus. (B) Histogram representing the number of loci linking to each gene at each of the 3 stages. The color code is the same as in A. (C) Plot showing the size of each iQTL at stages 2 (green) and 3 (red). The size of the circle is proportional to the number of genes linked to the iQTL. Both axes relate to chromosomal location with the position of the chromosome marked in bold.
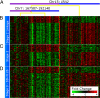
Fig. 3.
Gene expression in iQTL modules resemble environmental response. A heat map showing the _IRA2_–chrVII iQTL module and the expression of the genes linked at stages 2 and 3. Each row represents a gene, and each column represents a strain. The module is organized as a decision tree based on the strain's genotype and whether it inherited the BY (blue) or RM (purple) genotype for each of the interacting loci. (A) Top split based on the primary locus, chromosome XV:IRA2. The lower split is based on the secondary locus chromosome VII:167587–192140. (B) Eighty genes linked in stage 2. The columns represent strains and are arranged according to the tree; the vertical dotted yellow lines show the split point in the genotype. (C) Sixty-two genes linked in stage 3. The variance in expression of these genes is >0.25 SD. These genes were considered in stages 1 and 2, but did not pass the higher threshold for significance. (D) An additional 88 genes are linked in stage 3. These genes are not considered in stage 2 because their variance in expression is <0.25 SD and are hence noisier. The names of the genes represented in B–D are provided in
Table S3
.
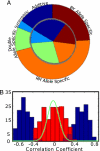
Fig. 4.
Landscape of genetic interactions between loci. (A) A pie chart representing the types of interactions between loci in our analysis. The outer circle represents genes, and the inner circle represents modules. RM allele-specific interactions are orange, BY allele-specific interactions are brown. Blue represents situations in which the secondary allele links to both sides, additive interactions are dark blue, and synergistic interactions are light blue. Green represents modules with 2 different allele-specific interactions, one for each side. The dominance of allele-specific interactions is evident. (B) Histogram of correlation coefficients in allele-specific modules. The data show that the effect of the secondary locus on the noninteracting allele is negligible. The x axis is the correlation coefficient between the secondary locus and the mean expression level for genes in the module. The y axis shows the number of modules. The blue bars represent data from the interacting primary allele and the red bars represent the other noninteracting allele. The green line shows that the distribution for randomly chosen pairs of loci is similar to the histogram in red demonstrating that the interactions are indeed with only one allele and not the other.
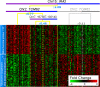
Fig. 5.
Allele-specific IRA2 module (see also
Fig. S6
). The IRA2-TCM62 iQTL module is graphically represented as described in Fig. 3. For compactness, representative genes were chosen for each pattern. The full list of genes for each pattern is provided in
Table S4
. We manually added an additional partition by using the chromosome VII locus from Fig. 3 to TCM62-BY to demonstrate that the chromosome VII locus represents an alternative pathway to that affected by TCM62-RM.
Similar articles
- The molecular basis of phenotypic variation in yeast.
Fay JC. Fay JC. Curr Opin Genet Dev. 2013 Dec;23(6):672-7. doi: 10.1016/j.gde.2013.10.005. Epub 2013 Nov 21. Curr Opin Genet Dev. 2013. PMID: 24269094 Free PMC article. Review. - Multiple locus linkage analysis of genomewide expression in yeast.
Storey JD, Akey JM, Kruglyak L. Storey JD, et al. PLoS Biol. 2005 Aug;3(8):e267. doi: 10.1371/journal.pbio.0030267. Epub 2005 Jul 26. PLoS Biol. 2005. PMID: 16035920 Free PMC article. - Dissecting the Genetic Regulation of Yeast Growth Plasticity in Response to Environmental Changes.
Zan Y, Carlborg Ö. Zan Y, et al. Genes (Basel). 2020 Oct 29;11(11):1279. doi: 10.3390/genes11111279. Genes (Basel). 2020. PMID: 33137976 Free PMC article. - Natural Variation in SER1 and ENA6 Underlie Condition-Specific Growth Defects in Saccharomyces cerevisiae.
Sirr A, Scott AC, Cromie GA, Ludlow CL, Ahyong V, Morgan TS, Gilbert T, Dudley AM. Sirr A, et al. G3 (Bethesda). 2018 Jan 4;8(1):239-251. doi: 10.1534/g3.117.300392. G3 (Bethesda). 2018. PMID: 29138237 Free PMC article. - Genetic mapping of quantitative phenotypic traits in Saccharomyces cerevisiae.
Swinnen S, Thevelein JM, Nevoigt E. Swinnen S, et al. FEMS Yeast Res. 2012 Mar;12(2):215-27. doi: 10.1111/j.1567-1364.2011.00777.x. Epub 2012 Jan 24. FEMS Yeast Res. 2012. PMID: 22150948 Review.
Cited by
- Harnessing natural sequence variation to dissect posttranscriptional regulatory networks in yeast.
Fazlollahi M, Lee E, Muroff I, Lu XJ, Gomez-Alcala P, Causton HC, Bussemaker HJ. Fazlollahi M, et al. G3 (Bethesda). 2014 Jun 17;4(8):1539-53. doi: 10.1534/g3.114.012039. G3 (Bethesda). 2014. PMID: 24938291 Free PMC article. - Variants in exons and in transcription factors affect gene expression in trans.
Kreimer A, Pe'er I. Kreimer A, et al. Genome Biol. 2013 Jul 11;14(7):R71. doi: 10.1186/gb-2013-14-7-r71. Genome Biol. 2013. PMID: 23844908 Free PMC article. - eQTL Epistasis - Challenges and Computational Approaches.
Huang Y, Wuchty S, Przytycka TM. Huang Y, et al. Front Genet. 2013 May 31;4:51. doi: 10.3389/fgene.2013.00051. eCollection 2013. Front Genet. 2013. PMID: 23755066 Free PMC article. - Harnessing gene expression to identify the genetic basis of drug resistance.
Chen BJ, Causton HC, Mancenido D, Goddard NL, Perlstein EO, Pe'er D. Chen BJ, et al. Mol Syst Biol. 2009;5:310. doi: 10.1038/msb.2009.69. Epub 2009 Oct 13. Mol Syst Biol. 2009. PMID: 19888205 Free PMC article. - A sexual ornament in chickens is affected by pleiotropic alleles at HAO1 and BMP2, selected during domestication.
Johnsson M, Gustafson I, Rubin CJ, Sahlqvist AS, Jonsson KB, Kerje S, Ekwall O, Kämpe O, Andersson L, Jensen P, Wright D. Johnsson M, et al. PLoS Genet. 2012;8(8):e1002914. doi: 10.1371/journal.pgen.1002914. Epub 2012 Aug 30. PLoS Genet. 2012. PMID: 22956912 Free PMC article.
References
- Brem RB, Yvert G, Clinton R, Kruglyak L. Genetic dissection of transcriptional regulation in budding yeast. Science. 2002;296:752–755. - PubMed
- Cheung VG, et al. Natural variation in human gene expression assessed in lymphoblastoid cells. Nat Genet. 2003;33:422–425. - PubMed
- Schadt EE, et al. Genetics of gene expression surveyed in maize, mouse, and man. Nature. 2003;422:297–302. - PubMed
- Yvert G, et al. Trans-acting regulatory variation in Saccharomyces cerevisiae and the role of transcription factors. Nat Genet. 2003;35:57–64. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases