The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth - PubMed (original) (raw)
The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth
Amanda J G Dickinson et al. Development. 2009 Apr.
Abstract
The primary mouth forms from ectoderm and endoderm at the extreme anterior of the embryo, a conserved mesoderm-free region. In Xenopus, a very early step in primary mouth formation is loss of the basement membrane between the ectoderm and endoderm. In an unbiased microarray screen, we defined genes encoding the sFRPs Frzb-1 and Crescent as transiently and locally expressed in the primary mouth anlage. Using antisense oligonucleotides and ;face transplants', we show that frzb-1 and crescent expression is specifically required in the primary mouth region at the time this organ begins to form. Several assays indicate that Frzb-1 and Crescent modulate primary mouth formation by suppressing Wnt signaling, which is likely to be mediated by beta-catenin. First, a similar phenotype (no primary mouth) is seen after loss of Frzb-1/Crescent function to that seen after temporally and spatially restricted overexpression of Wnt-8. Second, overexpression of either Frzb-1 or Dkk-1 results in an enlarged primary mouth anlage. Third, overexpression of Dkk-1 can restore a primary mouth to embryos in which Frzb-1/Crescent expression has been inhibited. We show that Frzb-1/Crescent function locally promotes basement membrane dissolution in the primary mouth primordium. Consistently, Frzb-1 overexpression decreases RNA levels of the essential basement membrane genes fibronectin and laminin, whereas Wnt-8 overexpression increases the levels of these RNAs. These data are the first to connect Wnt signaling and basement membrane integrity during primary mouth development, and suggest a general paradigm for the regulation of basement membrane remodeling.
Figures
Fig. 1.
Schematic summarizing microarray screen for genes whose expression is enriched in the primary mouth anlage. (A) Regions microdissected for RNA collection: anterior dorsal region (AD, dark gray), ventral region including the cement gland (CG+V, light gray) and the presumptive primary mouth (PMo, red). Foregut epithelium is shown in yellow. (B) Expression of frzb-1 and ef1-alpha in the primary mouth (red) relative to flanking regions (88%, microarray; 71%, qRT-PCR).
Fig. 2.
In situ hybridization of frzb-1 and crescent mRNA during neurula and early tailbud stages. (A-H′) In situ hybridization of frzb-1 (A-D) and crescent (E-H) during neurula and early tailbud stages. frzb-1 and crescent are stained purple/blue, the cement gland marker (XCG), dark red and the CNS-specific marker (nrp1), light orange. Arrows indicate the presumptive primary mouth. cg, cement gland. A-H are frontal views, scale bars: 200 μm; A′-H′ are sagittal sections with anterior to the left, scale bars: 130 μm.
Fig. 3.
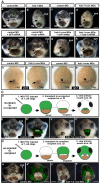
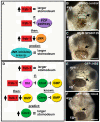
frzb-1/crescent loss-of-function analysis. (A)frzb-1 and crescent loss of function using antisense morpholinos, and rescue by frzb-1 mRNA in whole embryos. Frontal views are shown, assayed at stage 40 in two to four independent experiments. cg, cement gland. Scale bars: 250 μm. Open primary mouth, black dotted line; closed stomodeum, yellow dotted line. (a-d) Morpholino injection (60-80 ng/embryo). (a) Control morpholino results in a normal primary mouth (100%, _n_=121). (b) Two frzb-1 start site morpholinos result in a very small stomodeum (97%, _n_=57). (c) A crescent splice blocking morpholino results in a smaller primary mouth (100%, _n_=50). (d) frzb-1 morpholinos (15 ng/embryo of each) and crescent morpholino (30 ng/embryo) result in neither stomodeum nor primary mouth (99%,_n_=66). (e-h) Rescue: 60 ng morpholino and 200 pg mRNA was injected/embryo. (e) Control morpholinos plus GFP mRNA have no effect (100%,_n_=45). (f) Control morpholinos with frzb-1 mRNA results in a normal primary mouth (100%, _n_=33). (g)frzb-1/crescent morpholinos plus GFP mRNA result in neither a stomodeum nor a primary mouth (94%, _n_=32). (h)frzb-1/crescent morpholinos plus frzb-1 mRNA results in a primary mouth opening (86%, _n_=55). Arrows indicate the primary mouth or region where it would form. (B) The primary mouth anlage is correctly specified. (a,b) Analysis of pitx3 expression by in situ hybridization in (a) control and (b)frzb-1/crescent morphant embryos. Note that pitx3 expression is present in the morphant. Scale bars: 200 μm. (c,d) Analysis of vgl-2 expression by in situ hybridization in (c) control and (d) frzb-1/crescent morphant embryos. Note that vgl-2 expression it is present in the morphant. Arrows indicate the primary mouth anlage. Scale bars: 200 μm. (C) Localizing morphant and wild-type tissue using face transplants. (a) Schematic of experimental design: donor morphant tissue (FITC labeled) was transplanted to uninjected sibling recipients. (b) The primary mouth is normal when donor tissue is derived from embryos injected with control morpholinos (100%, n_=9). (b′) Overlay of b with FITC fluorescence, indicating the location of the donor tissue in the recipient. (c) When donor tissue is derived from_frzb-1/crescent morphants, 83% of recipients do not form a primary mouth opening and 17% form a small stomodeum (_n_=12). These embryos also have abnormalities in surrounding tissues, pigment cells do not migrate normally and the face appears thinner. (c′) Overlay of c with FITC fluorescence. (d) Schematic of experimental design: donor wild-type tissue was transplanted to morphants. (e) The primary mouth is normal when recipients are injected with standard control morpholinos (100%, _n_=7). (e′) Overlay of e with FITC fluorescence. (f) When recipients are frzb-1/crescent morphants, a primary mouth is present (80%, _n_=15). Although the primary mouth is not a normal shape, a deep invagination forms, followed by perforation. (f′) Overlay of f with FITC fluorescence.
Fig. 4.
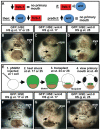
Temporal and spatial overexpression of wnt-8 under control of a heat-shock promoter element (HSE) and using ectoderm transplants. Frontal views are shown, assayed at stage 40 in two to three independent experiments. Open primary mouth, black dotted line; cg, cement gland; pSGH2, ISceI-GFP-HSE plasmid. Arrows indicate the primary mouth or region where it would form. Scale bars: 250 μm. (A) Schematic showing prediction that if Frzb-1/Cresent inhibits Wnt signaling, increased Wnt-8 would phenocopy loss of Frzb-1/Crescent. (B) Injected with GFP::HSE followed by heat shock at either stage 17 or 25 has no effect on primary mouth formation (90%,_n_=50). (C) Injection of GFP::HSE::wnt-8 and heat shock administered at stage 17 results in neither a stomodeum nor a primary mouth (97%, _n_=65). (D) Injection of GFP::HSE::wnt-8 and heat shock administered later (stage 25-28) results in a normal primary mouth (47%) or a slightly smaller one (53%, _n_=32). (E) Schematic of experimental design using ectoderm transplants. (F) Control (GFP::HSE): when heat shock was administered at stage 17 and transplants performed at stage 24, recipients form a primary mouth (100%, _n_=12). The same is true if heat shock is administered at stage 24 and transplants are performed at stage 28 (_n_=10). (G) GFP::HSE::wnt-8: when heat shock was administered at stage 17 and transplants performed at stage 24, 83% of the recipients do not form a stomodeum or a primary mouth (_n_=12). (H) When the experiment in G was performed later, 88% formed a normal primary mouth (_n_=9).
Fig. 5.
Frzb-1 and Dkk-1 regulate the same pathway. (A) Temporal overexpression of frzb-1 and dkk-1 during neurula (stage 17). b′,d′,e′ are sagittal optical sections (anterior to the left). Assayed at stage 40, two independent experiments. Open primary mouth, black dotted line; closed stomodeum, yellow dotted line; bracket indicates the position of the primary mouth or stomodeum; pSGH2, ISceI-GFP-HSE plasmid. Scale bars: 250 μm. (a) Schematic of experimental design. (b) Control (GFP::HSE) results in a normal primary mouth (90%, _n_=66), frontal view. (b′) Embryo treated as in b with a normal primary mouth. (c) Schematic showing the prediction that increased Frzb-1 would mimic overexpression of Dkk-1. (d) Injection of GFP::HSE::frzb-1 results in a larger stomodeum, frontal view (48%, _n_=50). (d′) Embryo treated as in d, showing increased stomodeal surface. (e) Injection of GFP::HSE::dkk1 results in a larger stomodeum, frontal view (53%, n_=38). (e′) Embryo treated as in e has an increased stomodeal surface. (B) Rescue of the primary mouth in_frzb-1/crescent morphants with dkk-1 mRNA. Frontal views, assayed at stage 40 in duplicate. Black arrow, primary mouth; cg, cement gland. Scale bars: 250 μm. (a) Schematic of experimental design. (b) The primary mouth is absent when donor tissue is derived from embryos injected with frzb-1/crescent morpholinos and GFP mRNA (90%, n_=10). (b′) Overlay of b with FITC fluorescence, indicating the location of the donor tissue in the recipient. (c) When donor tissue is derived from embryos injected with_frzb-1/crescent morpholinos and dkk-1 mRNA, 66% of recipients form a primary mouth and 33% form a large stomodeum with no opening (_n_=12). (c′) Overlay of c with FITC fluorescence.
Fig. 6.
Frzb-1 does not regulate Wnt/PCP or Bmp signaling. Frontal views, assayed at stage 40, from 2-3 independent experiments. Open primary mouth, black dotted line; closed stomodeum, yellow dotted; cg, cement gland. Black arrows indicate the primary mouth or region where it would form. Scale bars: 250 μm. (A) Schematic showing the prediction that if frzb-1 inhibits the PCP pathway, JNK inhibition would mimic frzb-1 overexpression. (B) Treatment with 1% DMSO results in a normal primary mouth (100%, _n_=15). (C) Treatment with 20 μm SP600125 results in neither a stomodeum nor a primary mouth (100%, _n_=15). (D) Schematic showing the prediction that if frzb-1 inhibits BMP signaling, increased Chordin would phenocopy frzb-1 overexpression. (E) The control GFP::HSE results in a normal primary mouth (100%, _n_=30). (F) GFP::HSE:chrd results in a small stomodeum (63%, _n_=32).
Fig. 7.
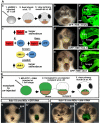
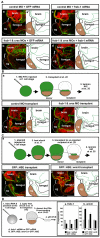
Laminin persists in the primary mouth region when Frzb-1/Crescent are depleted or when wnt-8 is overexpressed. Sagittal sections (anterior to the left) assayed at stage 35-37, in 2-3 independent experiments. Laminin is immunolabed green, nuclear propidium iodide, red; cement gland is outlined by a dotted gray line. Panels denoted by primes are tracings of Laminin immunolabeling (green). Bracket indicates the presumptive primary mouth; cg, cement gland. Scale bars: 170 μm. (A) Laminin persistence in frzb-1/crescent morphants is specific. (a) Control morpholino and GFP mRNA results in a normal absence of Laminin in the presumptive primary mouth (100%, n_=10). (b) In_frzb-1/crescent morphants (also injected with control GFP mRNA), Laminin persists in the primary mouth region (87%, _n_=10). Note that similar phenotypes were seen in morphants injected without GFP mRNA. (c) Control morpholino and frzb-1 mRNA (200 pg) results in a normal absence of Laminin in the presumptive primary mouth (n_=10). (d) Co-injection of frzb-1/crescent morpholinos and_frzb-1 mRNA restores the absence of Laminin in 70% of morphants (n_=10). (B) Laminin persists in embryos locally depleted of_frzb-1/crescent. (a) Schematic of the experimental design. (b) Recipients receiving tissue injected with control morpholino and GFP mRNA have a normal absence of Laminin (100%, n_=7). (c) Eighty-nine percent of recipients receiving tissue from_frzb-1/crescent morphants have persistent Laminin (_n_=9). (C) Temporal and spatial overexpression of Wnt-8 results in persistent Laminin. (a) Schematic of the experimental design. (b) Recipients receiving tissue injected with GFP::HSE have a normal absence of Laminin (91%, n_=11). (c) Seventy-five percent of recipients receiving tissue from embryos injected with GFP::HSE::wnt-8 have persistent Laminin (n_=12). (D) Wnt signaling regulates the expression of basement membrane components. Schematic depicts experimental design. Results are an average of two independent experiments. (a) Increased frzb-1 results in_fibronectin mRNA that is 45% of the control level,laminin_-γ_1 mRNA that is 48% of the control level and β_1-integrin that is 112% of the control level (n_=20). (b) Temporally increased wnt-8 results in fibronectin mRNA that is 232% of the control level, laminin_-γ_1 mRNA that is 170% of the control level and β_1-integrin mRNA that is 93% of the control levels (_n_=20).
Fig. 8.
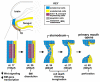
Model of frzb-1/crescent function in primary mouth formation. At neurula stages (stage 17-22), frzb-1 and_crescent_ are expressed in a subset of cells that will form the primary mouth (purple). In these cells, frzb-1 and crescent inhibit Wnt signaling, which in turn prevents the continued synthesis of proteins Laminin and Fibronectin. Without such proteins, the basement membrane integrity is lost and by early tailbud stages (stage 24-26) it disappears. The dissolution of the basement membrane leads to subsequent stages of primary mouth development including cell death, invagination, thinning and finally perforation. frzb-1/_crescent_-expressing cells are colored purple; ectoderm, blue; endoderm, yellow. The basement membrane (BM) is indicated by a black line; cg, cement gland.
Similar articles
- Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range.
Mii Y, Taira M. Mii Y, et al. Development. 2009 Dec;136(24):4083-8. doi: 10.1242/dev.032524. Epub 2009 Nov 11. Development. 2009. PMID: 19906850 - A direct screen for secreted proteins in Xenopus embryos identifies distinct activities for the Wnt antagonists Crescent and Frzb-1.
Pera EM, De Robertis EM. Pera EM, et al. Mech Dev. 2000 Sep;96(2):183-95. doi: 10.1016/s0925-4773(00)00394-4. Mech Dev. 2000. PMID: 10960783 - Wnt antagonism initiates cardiogenesis in Xenopus laevis.
Schneider VA, Mercola M. Schneider VA, et al. Genes Dev. 2001 Feb 1;15(3):304-15. doi: 10.1101/gad.855601. Genes Dev. 2001. PMID: 11159911 Free PMC article. - Mouth development.
Chen J, Jacox LA, Saldanha F, Sive H. Chen J, et al. Wiley Interdiscip Rev Dev Biol. 2017 Sep;6(5):e275. doi: 10.1002/wdev.275. Epub 2017 May 17. Wiley Interdiscip Rev Dev Biol. 2017. PMID: 28514120 Free PMC article. Review. - Positioning the extreme anterior in Xenopus: cement gland, primary mouth and anterior pituitary.
Dickinson A, Sive H. Dickinson A, et al. Semin Cell Dev Biol. 2007 Aug;18(4):525-33. doi: 10.1016/j.semcdb.2007.04.002. Epub 2007 Apr 19. Semin Cell Dev Biol. 2007. PMID: 17509913 Review.
Cited by
- Quantification of orofacial phenotypes in Xenopus.
Kennedy AE, Dickinson AJ. Kennedy AE, et al. J Vis Exp. 2014 Nov 6;(93):e52062. doi: 10.3791/52062. J Vis Exp. 2014. PMID: 25407252 Free PMC article. - Wnt signaling in orofacial clefts: crosstalk, pathogenesis and models.
Reynolds K, Kumari P, Sepulveda Rincon L, Gu R, Ji Y, Kumar S, Zhou CJ. Reynolds K, et al. Dis Model Mech. 2019 Feb 4;12(2):dmm037051. doi: 10.1242/dmm.037051. Dis Model Mech. 2019. PMID: 30760477 Free PMC article. Review. - Amphioxus mouth after dorso-ventral inversion.
Kaji T, Reimer JD, Morov AR, Kuratani S, Yasui K. Kaji T, et al. Zoological Lett. 2016 Feb 6;2:2. doi: 10.1186/s40851-016-0038-3. eCollection 2016. Zoological Lett. 2016. PMID: 26855789 Free PMC article. - Using frogs faces to dissect the mechanisms underlying human orofacial defects.
Dickinson AJ. Dickinson AJ. Semin Cell Dev Biol. 2016 Mar;51:54-63. doi: 10.1016/j.semcdb.2016.01.016. Epub 2016 Jan 15. Semin Cell Dev Biol. 2016. PMID: 26778163 Free PMC article. Review. - Development and evolution of the vertebrate primary mouth.
Soukup V, Horácek I, Cerny R. Soukup V, et al. J Anat. 2013 Jan;222(1):79-99. doi: 10.1111/j.1469-7580.2012.01540.x. Epub 2012 Jul 16. J Anat. 2013. PMID: 22804777 Free PMC article. Review.
References
- Agathon, A., Thisse, C. and Thisse, B. (2003). The molecular nature of the zebrafish tail organizer. Nature 424, 448-452. - PubMed
- Bajoghli, B., Aghaallaei, N., Heimbucher, T. and Czerny, T. (2004). An artificial promoter construct for heat-inducible misexpression during fish embryogenesis. Dev. Biol. 271, 416-430. - PubMed
- Bovolenta, P., Esteve, P., Ruiz, J. M., Cisneros, E. and Lopez-Rios, J. (2008). Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 121, 737-746. - PubMed
- Bradley, L., Sun, B., Collins-Racie, L., LaVallie, E., McCoy, J. and Sive, H. (2000). Different activities of the frizzled-related proteins frzb2 and sizzled2 during Xenopus anteroposterior patterning. Dev. Biol. 227, 118-132. - PubMed
- Carmona-Fontaine, C., Acuna, G., Ellwanger, K., Niehrs, C. and Mayor, R. (2007). Neural crests are actively precluded from the anterior neural fold by a novel inhibitory mechanism dependent on Dickkopf1 secreted by the prechordal mesoderm. Dev. Biol. 309, 208-221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous