Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function - PubMed (original) (raw)
Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function
Jifang Tao et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Mutations of MECP2 (Methyl-CpG Binding Protein 2) cause Rett syndrome. As a chromatin-associated multifunctional protein, how MeCP2 integrates external signals and regulates neuronal function remain unclear. Although neuronal activity-induced phosphorylation of MeCP2 at serine 421 (S421) has been reported, the full spectrum of MeCP2 phosphorylation together with the in vivo function of such modifications are yet to be revealed. Here, we report the identification of several MeCP2 phosphorylation sites in normal and epileptic brains from multiple species. We demonstrate that serine 80 (S80) phosphorylation of MeCP2 is critical as its mutation into alanine (S80A) in transgenic knock-in mice leads to locomotor deficits. S80A mutation attenuates MeCP2 chromatin association at several gene promoters in resting neurons and leads to transcription changes of a small number of genes. Calcium influx in neurons causes dephosphorylation at S80, potentially contributing to its dissociation from the chromatin. We postulate that phosphorylation of MeCP2 modulates its dynamic function in neurons transiting between resting and active states within neural circuits that underlie behaviors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
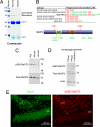
Identification of MeCP2 phosphorylation sites. (A) Coomassie staining of the SDS/PAGE gel showed MeCP2 purified from normal and seized rat brain extracts. (B) A summary of MeCP2 phosphorylation sites identified from normal and epileptic mouse and rat brain extracts. Sites marked in red are constitutively phosphorylated and sites marked in green are only phosphorylated in seized brain samples. (C) Phosphorylated MeCP2 at S80 was detected in human, mouse and rat brain extracts and (D) the phospho-S80 band was abolished upon phosphatase treatment. (E) Immunostaining of postnatal 3-week-old mouse hippocampal dentate gyrus (DG) region with phospho-S80 MeCP2 and neuronal marker NeuN.
Fig. 2.
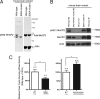
Mecp2S80A and Mecp2S421A;S424A knock-in mice showed, respectively, decreased and increased locomotor activities. (A) Phospho-S80 antibody detected a band from brain extracts of wild-type mice, but not the Mecp2S80A mice. The asterisk marks a nonspecific band. (B) Phospho-S421 antibody detected a band in normal wild-type mice brain extract, but not in brains of Mecp2S421A;S424A mice. (C) Mecp2S80A mice showed decreased locomotor activity when compared with their wild type littermates (n = 10 for wild type, n = 13 for S80A mutants, P = 0.029). Mecp2S421A;S424A mice showed increased locomotor activity when compared with their wild type littermates (n = 14 for wild type, n = 15 for S421AS424A mutants, P = 0.014).
Fig. 3.
Phosphorylations of MeCP2 at S80 and S421 are dynamically regulated in response to neuronal activity and do not cross-talk with each other. (A) Brain extracts of normal mice or mice that have undergone seizures induced by kainic acid were blotted first with phospho-S80 antibody and then MeCP2 full-length antibody. The asterisk marks the upper shifted band. (B and C) E15 + 3DIV cultured mouse cortical neurons were treated either with 0.5 μM tetrodotoxin (TTX) or 50 mM KCl for 1 h before harvested for protein assays. Western blot showed that upon depolarization, S80 phosphorylation decreased (B) while S421 phosphorylation increased (C) with phospho-serine 133-Creb as a positive control for depolarization. (D) E14 cultured Mecp2 −/y mouse cortical neurons were infected with lentiviruses expressing wild-type, serine 421 to alanine mutant (S421A) or serine 421 to aspartate mutant (S421D) MeCP2. Western blot was done with the antibody recognizing full-length MeCP2. (E) Flag tagged wild-type MeCP2, serine 80 to alanine mutant (S80A) and serines 80 to aspartate mutant (S80D) were expressed in E15 mouse cortical neurons by lentiviral infection. Protein lysates were blotted with flag antibody. Densitometry analysis of the upper band (phospho-S421 MeCP2) intensity versus both bands (total MeCP2) showed little difference (n = 3, 1-way ANOVA analysis, P = 0.839). (F) Similarly, neurons overexpressing flag-tagged MeCP2 were treated with TTX for 1 h before harvested for the protein lysates. Exogenous MeCP2 were immunoprecipitated with flag antibody and subjected to Western blot with phospho-S80 antibody and flag antibody respectively. Densitometry analysis of phospho-S80 intensity versus flag intensity showed that S421D did not change phospho-S80 intensity compared with wild-type MeCP2. S421A showed a slight but not significant increase of S80 phosphorylation (n = 3, 1-way ANOVA analysis, P = 0.205). (G) Nimodipine (5 μM) was applied in E15 mouse cortical neuronal culture for 1 h before depolarizing the neurons with 50 mM KCl. (H) E15 mouse cortical neurons were treated with KN62 (5 μM) for 1 h before depolarization with 50 mM KCl for 1 h and subsequently underwent Western blot. (I) SMARTPOOL CaMK IV siRNA and control siRNA (Dharmacon) were transfected into E15 mouse cortical neurons with nucleofector (Amaxa). Protein lysates were harvested 72 h after transfection and undergone Western blot. (J) Densitometry quantification of Western blots showed CaMK IV knockdown decreased S421 phosphorylation by 50% (P < 0.05, n = 3, paired t test).
Fig. 4.
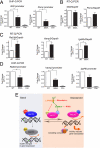
MeCP2 S80A mutation attenuated chromatin association affinity at candidate gene promoters and caused subtle gene expression changes. E15 mouse cortical neurons were infected with lentiviruses overexpressing flag-tagged or HA-tagged wild-type and S80A MeCP2. (A and D) Chromatin immunoprecipitation followed by quantitative PCR showed that at Gtl2 and Pomc promoters (A) and Rab3d, Vamp3, and Igsf4b promoters (D), serine 80 to alanine mutation weakened MeCP2 association with chromatin. IP/WCE values have been normalized with flag protein expression level (t test results: Gtl2 n = 6, P = 0.008; Pomc n = 7, P = 0.005; Vamp3 n = 3, P = 0.002; Rab3d n = 3, P = 0.046). (B and C) RNA was extracted from E15 + 3DIV Mecp2 −/y mouse cortical neurons infected with lentiviruses expressing wild-type and S80A MeCP2 respectively and subjected to quantitative RT-PCR. Among the candidate binding targets, Igsf4b, Rab3d and Vamp3 (C) showed significant up-regulation in MeCP2 S80A samples compared with wild-type samples (n = 5, P < 0.05, t test), whereas Gtl2 and Pomc (B) did not show obvious changes in gene transcription. (E) A hypothetical model of how MeCP2 phosphorylation and dephosphorylation may be part of the neuronal switch mechanism between resting and activated states.
Comment in
- The yin and yang of MeCP2 phosphorylation.
Chao HT, Zoghbi HY. Chao HT, et al. Proc Natl Acad Sci U S A. 2009 Mar 24;106(12):4577-8. doi: 10.1073/pnas.0901518106. Epub 2009 Mar 17. Proc Natl Acad Sci U S A. 2009. PMID: 19293386 Free PMC article. No abstract available.
Similar articles
- MECP2 Increases the Pro-Inflammatory Response of Microglial Cells and Phosphorylation at Serine 423 Regulates Neuronal Gene Expression upon Neuroinflammation.
Wittrahm R, Takalo M, Marttinen M, Kuulasmaa T, Mäkinen P, Kemppainen S, Martiskainen H, Rauramaa T, Pike I, Leinonen V, Natunen T, Haapasalo A, Hiltunen M. Wittrahm R, et al. Cells. 2021 Apr 9;10(4):860. doi: 10.3390/cells10040860. Cells. 2021. PMID: 33918872 Free PMC article. - Phosphorylation of distinct sites in MeCP2 modifies cofactor associations and the dynamics of transcriptional regulation.
Gonzales ML, Adams S, Dunaway KW, LaSalle JM. Gonzales ML, et al. Mol Cell Biol. 2012 Jul;32(14):2894-903. doi: 10.1128/MCB.06728-11. Epub 2012 May 21. Mol Cell Biol. 2012. PMID: 22615490 Free PMC article. - Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function.
Cohen S, Gabel HW, Hemberg M, Hutchinson AN, Sadacca LA, Ebert DH, Harmin DA, Greenberg RS, Verdine VK, Zhou Z, Wetsel WC, West AE, Greenberg ME. Cohen S, et al. Neuron. 2011 Oct 6;72(1):72-85. doi: 10.1016/j.neuron.2011.08.022. Neuron. 2011. PMID: 21982370 Free PMC article. - MeCP2 phosphorylation in the brain: from transcription to behavior.
Damen D, Heumann R. Damen D, et al. Biol Chem. 2013 Dec;394(12):1595-605. doi: 10.1515/hsz-2013-0193. Biol Chem. 2013. PMID: 23912219 Review. - MeCP2: the long trip from a chromatin protein to neurological disorders.
Ausió J, Martínez de Paz A, Esteller M. Ausió J, et al. Trends Mol Med. 2014 Sep;20(9):487-98. doi: 10.1016/j.molmed.2014.03.004. Epub 2014 Apr 21. Trends Mol Med. 2014. PMID: 24766768 Review.
Cited by
- Genetic syndromes caused by mutations in epigenetic genes.
Berdasco M, Esteller M. Berdasco M, et al. Hum Genet. 2013 Apr;132(4):359-83. doi: 10.1007/s00439-013-1271-x. Epub 2013 Jan 31. Hum Genet. 2013. PMID: 23370504 Review. - MeCP2 and the enigmatic organization of brain chromatin. Implications for depression and cocaine addiction.
Ausió J. Ausió J. Clin Epigenetics. 2016 May 21;8:58. doi: 10.1186/s13148-016-0214-5. eCollection 2016. Clin Epigenetics. 2016. PMID: 27213019 Free PMC article. Review. - Epigenetics and the regulation of stress vulnerability and resilience.
Zannas AS, West AE. Zannas AS, et al. Neuroscience. 2014 Apr 4;264:157-70. doi: 10.1016/j.neuroscience.2013.12.003. Epub 2013 Dec 13. Neuroscience. 2014. PMID: 24333971 Free PMC article. Review. - DNA methylation and methyl-CpG binding proteins: developmental requirements and function.
Bogdanović O, Veenstra GJ. Bogdanović O, et al. Chromosoma. 2009 Oct;118(5):549-65. doi: 10.1007/s00412-009-0221-9. Epub 2009 Jun 9. Chromosoma. 2009. PMID: 19506892 Free PMC article. Review. - Multiomics of early epileptogenesis in mice reveals phosphorylation and dephosphorylation-directed growth and synaptic weakening.
Hurtado Silva M, van Waardenberg AJ, Mostafa A, Schoch S, Dietrich D, Graham ME. Hurtado Silva M, et al. iScience. 2024 Mar 19;27(4):109534. doi: 10.1016/j.isci.2024.109534. eCollection 2024 Apr 19. iScience. 2024. PMID: 38600976 Free PMC article.
References
- Soderling TR. CaM-kinases: Modulators of synaptic plasticity. Curr Opin Neurobiol. 2000;10:375–380. - PubMed
- Frankland PW, O'Brien C, Ohno M, Kirkwood A, Silva AJ. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–313. - PubMed
- Wei F, et al. Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci. 2002;5:573–579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials