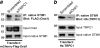Native Store-operated Ca2+ Influx Requires the Channel Function of Orai1 and TRPC1 - PubMed (original) (raw)
Native Store-operated Ca2+ Influx Requires the Channel Function of Orai1 and TRPC1
Min Seuk Kim et al. J Biol Chem. 2009.
Abstract
With the discovery of STIM1 and Orai1 and gating of both TRPC and Orai1 channels by STIM1, a central question is the role of each of the channels in the native store-operated Ca(2+) influx (SOCs). Here, we used a strategy of knockdown of Orai1 and of TRPC1 alone and in combination and rescue by small interfering RNA-protected mutants (sm) of smOrai1 and smTRPC1 to demonstrate that in human embryonic kidney (HEK) cells, rescue of SOCs required co-transfection of low levels of both smOrai1 and smTRPC1. The pore mutant Orai1(E106Q) failed to rescue the SOCs in the presence or absence of TRPC1 and, surprisingly, the pore mutant TRPC1(F562A) failed to rescue the SOCs in the presence or absence of Orai1. TRPC1 is gated by electrostatic interaction between TRPC1(D639D,D640D) with STIM1(K684K, K685K). Strikingly, the channel-dead TRPC1(D639K,D640K) that can be rescued only by the STIM1(K684E,K685E) mutant could restore SOCs only when expressed with Orai1 and STIM1(K684E,K685E). Accordingly, we found a mutual requirement of Orai1 and TRPC1 for their interaction with the native STIM1 in HEK cells. By contrast, SOC and the CRAC current in Jurkat cells were inhibited by knockdown of Orai1 but were not influenced by knockdown on TRPC1 or TRPC3. These findings define the molecular makeup of the native SOCs in HEK cells and the role of a STIM1-Orai1-TRPC1 complex in SOC activity.
Figures
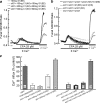
FIGURE 1.
The channel function of Orai1 is required for the native SOCs. a shows analysis of the protein level of FLAG-mCherry-tagged Orai1, Orai1 with silent mutation (smOrai1), and the mutant Orai1(E106Q) with silent mutation (smOrai1(E106Q)) in cells treated with scrambled or with Orai1 siRNA (siOrai1) and transfected with 100 ng of cDNA of the respective constructs. Tubulin levels are given as loading and specificity controls. b shows measurement of SOC activity in cells treated with scrambled siRNA (black) or siOrai1 (all other conditions) for 48 h and transfected with 100 ng/dish of vector (dashed black trace), mCherry-tagged smOrai1 (gray) or smOrai1(E106Q) (dashed gray trace). After an additional 24 h, the cells were used to measure SOC activity. Stores were depleted by perfusion with 25 μ
m
cyclopiazonic acid (CPA) in Ca2+-free medium containing 1 m
m
EGTA for 7.5 min, and Ca2+ influx was measured using medium containing 2 m
m
Ca2+. c shows the mean ± S.E. of the indicated number of cells from 4 experiments. d shows the store-independent current recorded with pipette solution containing 70 n
m
Ca2+ buffered with 5 m
m
EGTA (○). The store-dependent current was recorded with pipette solution containing 140 m
m
Cs+, 10 m
m
BAPTA, and 30 μ
m
inositol 1,4,5-trisphosphate (IP3). Bath solution was the same for all conditions and contained 10 m
m
Ca2+ and then was divalent-free medium supplemented with 5 m
m
EGTA. Cells were treated with scrambled Orai1 (▪) or siOrai1 (▵) and transfected with 100 ng of smOrai1 (•). Current recording started about 20 s after break-in. The I/V plots are from the peak current of each condition. The_black trace_ is the Orai1-dependent current obtained by subtracting the current recorded in cells treated with siOrai1 from the current recorded in cells treated with scrambled siRNA. The columns show the mean ± S.E. of the indicated number of experiments. e shows example traces, I/V plots, and the mean ± S.E. of the CRAC-like current found in 4/21 cells treated with scrambled siRNA, 3/17 cells treated with siTRPC1, 4/19 cells treated with siOrai1, and 4/18 cells treated with siTRPC1 + siOrai1.
FIGURE 2.
TRPC1 is required for the native SOCs. The upper blot of_a_ shows TRPC1 RT-PCR of HEK cells treated with siTRPC1. The lower blots show analysis of protein levels of HA-tagged TRPC1, TRPC1(F562A), TRPC1 with silent mutation (smTRPC1), and smTRPC1(F562A) in cells treated with scrambled or with TRPC1 siRNA (siTRPC1) and transfected with 100 ng of cDNA of the respective constructs. Tubulin levels are given as loading and specificity controls. b shows measurement of SOC activity in cells treated with scrambled siRNA (black), siTRPC1 (light gray), and transfected with smTRPC1 (gray) or treated with siTRPC1 + siOrai1 for 48 h (all other conditions) and transfected with enhanced green fluorescent protein and 100 ng/dish of vector (dashed gray trace), 100 ng of smOrai1 (dashed black trace), or 100 ng of smTRPC1 (light gray trace). After 24 h, cells were used to measure SOC activity by store depletion in Ca2+-free media and the readdition of 2 m
m
Ca2+ to the medium, as in Fig. 1. CPA, cyclopiazonic acid. c shows the mean ± S.E. of the indicated number of cells from at least 4 experiments.YFP, yellow fluorescent protein. d shows the current recorded with pipette solution containing 140 m
m
CsCl, 5 m
m
EGTA, and 1.5 m
m
CaCl2 to clamp free Ca2+ at 70 n
m
and bath solution containing 140 m
m
Na+ or NMDG+ and 0.5 m
m
EGTA. Cells were treated with scrambled TRPC1 (○, •) or siTRPC1 (□, ▵) and transfected with 100 ng of smTRPC1 (•, ▵). e shows the corresponding I/V at peak current of each condition. f shows the mean ± S.E. of 4 of the experiments.
FIGURE 3.
The channel function of TRPC1 is required for the native SOCs. In_a_, cells transfected with TRPC1(F562A) (light gray trace) for 24 h were used to measure SOCs-mediated Ca2+ influx as in Fig. 1. Cells were also treated with scrambled siRNA (black) or siTRPC1 + siOrai1 (dashed light gray and gray traces) for 48 h and were then transfected with 100 ng of smOrai1 + smTRPC1 (gray trace) or smOrai1 + smTRPC1(F562A) (dashed black trace), and 24 h later, they were used to measure SOCs, as in Fig. 1. CPA, cyclopiazonic acid. b shows the mean ± S.E. of the indicated number of cells from 3 experiments. YFP, yellow fluorescent protein.
FIGURE 4.
The SOC function of TRPC1 and Orai1 are required for the native SOCs. In a, untreated cells were transfected with 100 ng of STIM1(K684E,K685E) alone (gray trace), or cells treated with siOrai1 (all remaining) were transfected with the combination of the mutants TRPC1(D639K,D640K) + STIM1(K684E,K685E) at 100 ng (dashed gray trace), 300 ng (dashed black trace), or 500 ng (black trace). After 24 h, the transfected cells were used to measure SOC activity. CPA, cyclopiazonic acid. In b, all cells were treated with siOrai1 and siTRPC1 for 48 h and then transfected with 100 ng each of smOrai1 (dashed gray trace), smTRPC1(D639K,D640K) (dashed black trace), smTRPC1(D639K,D640K) + STIM1(K684E,K685E) (gray trace), or smOrai1 + smTRPC1(D639K,D640K) + STIM1(K684E,K685E) (black trace). SOC activity was measured 24 h after transfection as in Fig. 1. c shows the mean ± S.E. of the indicated number of cells from 4 experiments.eGFP, enhanced green fluorescent protein.
FIGURE 5.
Mutual dependence of Orai1 and TRPC1 for interaction with STIM1. HEK cells treated with scrambled siRNA (a and b) and siTRPC1 (a) or siOrai1 (b) were transfected with 100 ng of mCherry-FLAG-Orai1 (a) or HA-TRPC1 (b) and were used to immunoprecipitate the native STIM1 and blot for co-immunoprecipitation (IP) of Orai1 (a, with anti-FFLAG) or TRPC1 (b, anti-HA). Also shown are the inputs for Orai1, TRPC1, and the native STIM1. Similar results were obtained in 3 experiments.
FIGURE 6.
TRPC1 and TRPC3 are not required for _I_crac and SOCs in Jurkat cells. In a and b, HEK (a) and Jurkat cells (b) were treated with scrambled (black traces) or Orai1 siRNA (gray traces) for 72 h or transfected with STIM1(K684A,K685A) for 24 h (dashed gray traces) and used to measure SOC activity. CPA, cyclopiazonic acid. The inset in_c_ shows TRPC3 RT-PCR of HEK cells treated with siTRPC3. In_c–g_, Jurkat cells were treated with scrambled siRNA (Scram) or siTRPC1 or siTRPC3 for 72 h (c–g) before measurement of SOC activity (c and d) or the CRAC current (e–g). f shows representative I/V plots, and_d_ and g show the mean ± S.E. of the indicated number of experiments.
Similar articles
- TRPC Channels in the SOCE Scenario.
Lopez JJ, Jardin I, Sanchez-Collado J, Salido GM, Smani T, Rosado JA. Lopez JJ, et al. Cells. 2020 Jan 5;9(1):126. doi: 10.3390/cells9010126. Cells. 2020. PMID: 31948094 Free PMC article. Review. - Contribution of TRPC1 and Orai1 to Ca(2+) entry activated by store depletion.
Cheng KT, Ong HL, Liu X, Ambudkar IS. Cheng KT, et al. Adv Exp Med Biol. 2011;704:435-49. doi: 10.1007/978-94-007-0265-3_24. Adv Exp Med Biol. 2011. PMID: 21290310 Free PMC article. Review. - Local Ca²+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca²+ signals required for specific cell functions.
Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Cheng KT, et al. PLoS Biol. 2011 Mar;9(3):e1001025. doi: 10.1371/journal.pbio.1001025. Epub 2011 Mar 8. PLoS Biol. 2011. PMID: 21408196 Free PMC article. - Store-operated Ca2+ Entry Mediated by Orai1 and TRPC1 Participates to Insulin Secretion in Rat β-Cells.
Sabourin J, Le Gal L, Saurwein L, Haefliger JA, Raddatz E, Allagnat F. Sabourin J, et al. J Biol Chem. 2015 Dec 18;290(51):30530-9. doi: 10.1074/jbc.M115.682583. Epub 2015 Oct 22. J Biol Chem. 2015. PMID: 26494622 Free PMC article. - Evidence that Orai1 does not contribute to store-operated TRPC1 channels in vascular smooth muscle cells.
Shi J, Miralles F, Kinet JP, Birnbaumer L, Large WA, Albert AP. Shi J, et al. Channels (Austin). 2017 Jul 4;11(4):329-339. doi: 10.1080/19336950.2017.1303025. Epub 2017 Mar 16. Channels (Austin). 2017. PMID: 28301277 Free PMC article.
Cited by
- Role of protein kinase C in the activation of store-operated Ca(2+) entry in airway smooth muscle cells.
Gao Y, Zou J, Geng S, Zheng J, Yang J. Gao Y, et al. J Huazhong Univ Sci Technolog Med Sci. 2012 Jun;32(3):303-310. doi: 10.1007/s11596-012-0053-3. Epub 2012 Jun 9. J Huazhong Univ Sci Technolog Med Sci. 2012. PMID: 22684549 - Orai1 interacts with STIM1 and mediates capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells.
Ng LC, Ramduny D, Airey JA, Singer CA, Keller PS, Shen XM, Tian H, Valencik M, Hume JR. Ng LC, et al. Am J Physiol Cell Physiol. 2010 Nov;299(5):C1079-90. doi: 10.1152/ajpcell.00548.2009. Epub 2010 Aug 25. Am J Physiol Cell Physiol. 2010. PMID: 20739625 Free PMC article. - Enhanced Ca²⁺ influx from STIM1-Orai1 induces muscle pathology in mouse models of muscular dystrophy.
Goonasekera SA, Davis J, Kwong JQ, Accornero F, Wei-LaPierre L, Sargent MA, Dirksen RT, Molkentin JD. Goonasekera SA, et al. Hum Mol Genet. 2014 Jul 15;23(14):3706-15. doi: 10.1093/hmg/ddu079. Epub 2014 Feb 20. Hum Mol Genet. 2014. PMID: 24556214 Free PMC article. - TRPC Channels in the SOCE Scenario.
Lopez JJ, Jardin I, Sanchez-Collado J, Salido GM, Smani T, Rosado JA. Lopez JJ, et al. Cells. 2020 Jan 5;9(1):126. doi: 10.3390/cells9010126. Cells. 2020. PMID: 31948094 Free PMC article. Review. - Contribution of TRPC1 and Orai1 to Ca(2+) entry activated by store depletion.
Cheng KT, Ong HL, Liu X, Ambudkar IS. Cheng KT, et al. Adv Exp Med Biol. 2011;704:435-49. doi: 10.1007/978-94-007-0265-3_24. Adv Exp Med Biol. 2011. PMID: 21290310 Free PMC article. Review.
References
- Petersen, O. H., Sutton, R., and Criddle, D. N. (2006) Cell Calcium 40 593–600 - PubMed
- Feske, S., Gwack, Y., Prakriya, M., Srikanth, S., Puppel, S. H., Tanasa, B., Hogan, P. G., Lewis, R. S., Daly, M., and Rao, A. (2006) Nature 441 179–185 - PubMed
- Parekh, A. B., and Putney, J. W., Jr. (2005) Physiol. Rev. 85 757–810 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA00266/DA/NIDA NIH HHS/United States
- R00 HL093297/HL/NHLBI NIH HHS/United States
- DE12309/DE/NIDCR NIH HHS/United States
- MH068830/MH/NIMH NIH HHS/United States
- DA10309/DA/NIDA NIH HHS/United States
- DK38938/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous