Distinct and essential morphogenic functions for wall- and lipo-teichoic acids in Bacillus subtilis - PubMed (original) (raw)
Distinct and essential morphogenic functions for wall- and lipo-teichoic acids in Bacillus subtilis
Kathrin Schirner et al. EMBO J. 2009.
Abstract
Teichoic acids (TAs) are anionic polymers that constitute a major component of the cell wall in most Gram-positive bacteria. Despite decades of study, their function has remained unclear. TAs are covalently linked either to the cell wall peptidoglycan (wall TA (WTA)) or to the membrane (lipo-TA (LTA)). We have characterized the key enzyme of LTA synthesis in Bacillus subtilis, LTA synthase (LtaS). We show that LTA is needed for divalent cation homoeostasis and that its absence has severe effects on cell morphogenesis and cell division. Inactivation of both LTA and WTA is lethal and comparison of the individual mutants suggests that they have differentiated roles in elongation (WTA) and division (LTA). B. subtilis has four ltaS paralogues and we show how their roles are partially differentiated. Two paralogues have a redundant role in LTA synthesis during sporulation and their absence gives a novel absolute block in sporulation. The crystal structure of the extracytoplasmic part of LtaS, solved at 2.4-A resolution, reveals a phosphorylated threonine residue, which provides clues about the catalytic mechanism and identifies the active site of the enzyme.
Figures
Figure 1
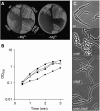
Deletion of ltaS suppresses the Mg2+ dependency of mbl mutants. (A) Growth of wild type (168), mbl mutant (2505), ltaS mutant (4283) and suppressed mbl mutant (Δ_mbl_ Δ_ltaS_, 4298) on NA plates with (left) or without (right) addition of 20 mM Mg2+. (B) Growth curves of wild type (168, ⧫), mbl mutant (2505, ▪), ltaS mutant (4283, ▴) and suppressed mbl mutant (Δ_mbl_ Δ_ltaS_, 4298, ○) in PAB medium at 37°C. (C) Phase-contrast microscopy of wild type (168), mbl mutant (2505), ltaS mutant (4283) and mbl ltaS double mutant (4298) grown in PAB medium at 37°C. Scale bar 5 μm.
Figure 2
Effect of metal ion concentration on the viability of wild type and ltaS mutants. (A) Growth of wild type (168) and ltaS mutant (strain 4286) on NA plates (left panel) containing 0.05 mM Mg2+ (middle panel) or 0.5 mM Mn2+ (right panel). The strains were grown to mid-exponential phase and spotted onto the plates in the dilutions as indicated. (B) Growth of ltaS mutant (strain 4283, left) and wild type (strain 168, right) on minimal medium plates containing 10, 100 and 500 μM Mg2+ as indicated.
Figure 3
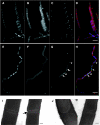
Effects of ltaS mutation on FtsZ ring formation, cell division and cell separation. The strains were grown to mid-exponential phase in PAB medium at 30°C (A–H) or 37°C (I, J). (A–H) Samples of wild type (strain 2020; A–D) and ltaS mutant (4605; E–H) carrying gfp-ftsZ under a xylose-inducible promoter were stained with DAPI (for DNA) and FM5-95 (for the membrane), and additionally imaged for FtsZ–GFP localization. (D, H) Overlays of the membrane (red), DNA (blue) and GFP (green) signals. Scale bar 5 μm. (I, J) Transmission electron microscopic images of transverse sections of two representative septa each of wild type (168; I) and ltaS mutant (strain 4284; J). Scale bar 100 nm.
Figure 4
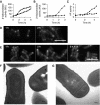
Effects of ltaS and yqgS deletion on sporulation. (A) Expression pattern of the spoIIA promoter measured by assaying β-galactosidase activity of strains 4614 (ltaS mutant, ▴) and 4615 (ltaS yqgS double mutant, •) grown in Schaeffer's medium at 37°C. (B) Measurements of β-galactosidase activity of strains ltaS mutants (4616, ▴) and ltaS yqgS double mutants (4617, •) carrying the lacZ gene under control of the σF-inducible spoIIQ promoter. (C) Assay for sporulation-specific alkaline phosphatase activity in ltaS mutant (4283, ▴) and ltaS yqgS double mutant (4611, •). All strains were grown in Schaeffer's medium at 37°C, time point 0 was set at the entry into stationary phase (A–C). (D, E) Localization of SpoIIE–GFP in an ltaS mutant background (strain 4618 (D)) and an ltaS yqgS double-mutant background (strain 4619 (E)). Time was measured from the point at which fluorescence of mCherry (under control of the spoIIA promoter) first became visible and images were taken at the time points indicated. Scale bar 5 μm. (F, G) Transmission electron microscopic images of sporulating cultures of ltaS mutant (4284 (F); asymmetric septum is marked ‘a', engulfed forespore is marked ‘b') and ltaS yqgS double mutant (4611 (G)). The strains were grown for 9 h in Schaeffer's medium at 37 before fixation. Scale bar 100 nm.
Figure 5
GFP–LtaS and GFP–YqgS localization. Fluorescence microscopy of strains 4607 and 4609 carrying inducible alleles of gfp-ltaS (A) and gfp-yqgS (B) at the amyE locus. Localization of GFP–YqgS in an spoIIE mutant background (strain 4626 (C)). Of each strain two fields are shown, all strains were grown in S-medium containing 0.5% xylose at 30°C. Scale bar 5 μm.
Figure 6
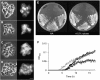
Morphology and viability of LTA and WTA mutants. (A–D) Morphology of a tagO mutant (strain 4282 (A)) and an ltaS tagO double mutant (strain 4621 (B)). Strain 4625 growing in the presence of 0.5% xylose (C) and after depleting for xylose (D). Left panels show phase-contrast microscopic images, right panels show staining of the membrane with FM5-95. The strains were grown in PAB medium with the addition of 10 mM Mg2+ at 37°C, scale bar 5 μm. (E) Strains 4622 (P_xyl_ -ltaS), 4623 (Δ_yfnI_ Δ_yqgS_ Δ_yvgJ P_ xyl -ltaS), 4624 (Δ_tagO P_ xyl -ltaS), 4625 (Δ_tagO_ Δ_yfnI_ Δ_yqgS_ Δ_yvgJ P_ xyl -ltaS) and wild type (168) were cultured on NA with (right) or without 0.5% xylose (left) overnight at 37°C. (F) Growth curve of strains 4624 (Δ_tagO P_ xyl -ltaS, ▴) and 4625 (Δ_tagO_ Δ_yfnI_ Δ_yqgS_ Δ_yvgJ P_ xyl -ltaS,  ) in PAB medium containing 10 mM Mg2+ with (closed symbols) or without (open symbols) addition of 0.5% xylose. The cultures were grown in a microtitre plate while shaking at 37°C.
) in PAB medium containing 10 mM Mg2+ with (closed symbols) or without (open symbols) addition of 0.5% xylose. The cultures were grown in a microtitre plate while shaking at 37°C.
Figure 7
Crystal structure of LtaS215–649. (A) The protein backbone is rendered as a secondary structure cartoon colour ramped from blue at the N terminus to red at the C terminus. Orthogonal views are shown with the secondary structure elements highlighted in the middle panel. (B) The active site of LtaS shown in two views, with residues coordinating the magnesium ion in the active site drawn as sticks with the final REFMAC-weighted _F_obs−_F_calc electron density map contoured at 1.5sigma and shown as blue wireframe. (C) The domain arrangement of full-length LtaS is illustrated schematically. The N-terminal, trans-membrane spanning helices are modelled, and coloured grey, in the context of a biological membrane. The structure of the C-terminal, catalytic domain of LtaS is drawn as a cartoon, colour-ramped in rainbow manner from N (blue) to C terminus (red). The location of the active site is highlighted by a dotted circle.
Similar articles
- Teichoic Acid Polymers Affect Expression and Localization of dl-Endopeptidase LytE Required for Lateral Cell Wall Hydrolysis in Bacillus subtilis.
Kasahara J, Kiriyama Y, Miyashita M, Kondo T, Yamada T, Yazawa K, Yoshikawa R, Yamamoto H. Kasahara J, et al. J Bacteriol. 2016 May 13;198(11):1585-1594. doi: 10.1128/JB.00003-16. Print 2016 Jun 1. J Bacteriol. 2016. PMID: 27002131 Free PMC article. - Phosphoglycerol-type wall and lipoteichoic acids are enantiomeric polymers differentiated by the stereospecific glycerophosphodiesterase GlpQ.
Walter A, Unsleber S, Rismondo J, Jorge AM, Peschel A, Gründling A, Mayer C. Walter A, et al. J Biol Chem. 2020 Mar 20;295(12):4024-4034. doi: 10.1074/jbc.RA120.012566. Epub 2020 Feb 11. J Biol Chem. 2020. PMID: 32047114 Free PMC article. - Discovery of genes required for lipoteichoic acid glycosylation predicts two distinct mechanisms for wall teichoic acid glycosylation.
Rismondo J, Percy MG, Gründling A. Rismondo J, et al. J Biol Chem. 2018 Mar 2;293(9):3293-3306. doi: 10.1074/jbc.RA117.001614. Epub 2018 Jan 17. J Biol Chem. 2018. PMID: 29343515 Free PMC article. - The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus.
Xia G, Kohler T, Peschel A. Xia G, et al. Int J Med Microbiol. 2010 Feb;300(2-3):148-54. doi: 10.1016/j.ijmm.2009.10.001. Epub 2009 Nov 6. Int J Med Microbiol. 2010. PMID: 19896895 Review. - Controlling biofilm and virulence properties of Gram-positive bacteria by targeting wall teichoic acid and lipoteichoic acid.
Jeong GJ, Khan F, Tabassum N, Cho KJ, Kim YM. Jeong GJ, et al. Int J Antimicrob Agents. 2023 Oct;62(4):106941. doi: 10.1016/j.ijantimicag.2023.106941. Epub 2023 Aug 2. Int J Antimicrob Agents. 2023. PMID: 37536571 Review.
Cited by
- Structural basis for synthase activation and cellulose modification in the E. coli Type II Bcs secretion system.
Anso I, Zouhir S, Sana TG, Krasteva PV. Anso I, et al. Nat Commun. 2024 Oct 11;15(1):8799. doi: 10.1038/s41467-024-53113-8. Nat Commun. 2024. PMID: 39394223 Free PMC article. - Comprehensive double-mutant analysis of the Bacillus subtilis envelope using double-CRISPRi.
Koo BM, Todor H, Sun J, van Gestel J, Hawkins JS, Hearne CC, Banta AB, Huang KC, Peters JM, Gross CA. Koo BM, et al. bioRxiv [Preprint]. 2024 Aug 16:2024.08.14.608006. doi: 10.1101/2024.08.14.608006. bioRxiv. 2024. PMID: 39185233 Free PMC article. Preprint. - The mycobacterial glycoside hydrolase LamH enables capsular arabinomannan release and stimulates growth.
Franklin A, Salgueiro VC, Layton AJ, Sullivan R, Mize T, Vázquez-Iniesta L, Benedict ST, Gurcha SS, Anso I, Besra GS, Banzhaf M, Lovering AL, Williams SJ, Guerin ME, Scott NE, Prados-Rosales R, Lowe EC, Moynihan PJ. Franklin A, et al. Nat Commun. 2024 Jul 9;15(1):5740. doi: 10.1038/s41467-024-50051-3. Nat Commun. 2024. PMID: 38982040 Free PMC article. - Lipoteichoic acids influence cell shape and bacterial division of Streptococcus suis serotype 2, but play a limited role in the pathogenesis of the infection.
Payen S, Giroux MC, Gisch N, Schombel U, Fittipaldi N, Segura M, Gottschalk M. Payen S, et al. Vet Res. 2024 Mar 19;55(1):34. doi: 10.1186/s13567-024-01287-w. Vet Res. 2024. PMID: 38504299 Free PMC article. - Lipoarabinomannan mediates localized cell wall integrity during division in mycobacteria.
Sparks IL, Kado T, Prithviraj M, Nijjer J, Yan J, Morita YS. Sparks IL, et al. Nat Commun. 2024 Mar 11;15(1):2191. doi: 10.1038/s41467-024-46565-5. Nat Commun. 2024. PMID: 38467648 Free PMC article.
References
- Archibald AR, Armstrong JJ, Baddiley J, Hay JB (1961) Teichoic acids and the structure of bacterial walls. Nature 191: 570–572 - PubMed
- Arigoni F, Pogliano K, Webb CD, Stragier P, Losick R (1995) Localization of protein implicated in establishment of cell type to sites of asymmetric division. Science 270: 637–640 - PubMed
- Barak I, Behari J, Olmedo G, Guzman P, Brown DP, Castro E, Walker D, Westpheling J, Youngman P (1996) Structure and function of the Bacillus SpoIIE protein and its localization to sites of sporulation septum assembly. Mol Microbiol 19: 1047–1060 - PubMed
- Bhavsar AP, Brown ED (2006) Cell wall assembly in Bacillus subtilis: how spirals and spaces challenge paradigms. Mol Microbiol 60: 1077–1090 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases