Use of tumour-responsive T cells as cancer treatment - PubMed (original) (raw)
Review
Use of tumour-responsive T cells as cancer treatment
Mary L Disis et al. Lancet. 2009.
Abstract
The stimulation of a tumour-specific T-cell response has several theoretical advantages over other forms of cancer treatment. First, T cells can home in to antigen-expressing tumour deposits no matter where they are located in the body-even in deep tissue beds. Additionally, T cells can continue to proliferate in response to immunogenic proteins expressed in cancer until all the tumour cells are eradicated. Finally, immunological memory can be generated, allowing for eradication of antigen-bearing tumours if they reoccur. We will highlight two direct methods of stimulating tumour-specific T-cell immunity: active immunisation with cancer vaccines and infusion of competent T cells via adoptive T-cell treatment. Preclinical and clinical studies have shown that modulation of the tumour microenvironment to support the immune response is as important as stimulation of the most appropriate effector T cells. The future of T-cell immunity stimulation to treat cancer will need combination approaches focused on both the tumour and the T cell.
Conflict of interest statement
Conflict of interest statement
MLD is an inventor on patents held by the University of Washington pertaining to T-cell therapies. EMJ has been a consultant for Amplimmune, where she provides advice on new agent development (pre-clinical advice to date); has been a consultant for Bristol-Myers Squibb in the past year; has licensed intellectual property to Anza Corp and has the potential to receive royalties in the future; and although she does not receive any financial support or has the potential for royalties, John Hopkins has the potential to receive royalties from Cell Geneys on the GVAX vaccine in the future. HB declares that she has no conflict of interest.
Figures
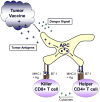
Figure 1. Tumour vaccine cells genetically modified to produce danger stimuli (similar to granulocyte-macrophage colony-stimulating factor) to attract and mature antigen-presenting cells
Tumour-associated antigens are shed from dying vaccine cells to provide a source of antigen. Antigens can then be taken up by antigen-presenting cells, expressing co-stimulatory molecules such as B7-1, to be processed and presented to T cells. Phenotypes of CD4+ T cells can either enhance (eg, Th1) or inhibit (eg, Treg) the immune response.
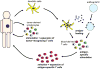
Figure 2. Isolation and ex-vivo stimulation of antigen-specific tumour-reactive T lymphocytes for adoptive transfer
Autologous lymphocytes are stimulated ex vivo with antigen-loaded antigen-presenting cells, such as dendritic cells. Enriched tumour-specific T cells are then re-infused for treatment.
Similar articles
- Broadly expressed tumour-associated proteins as targets for cytotoxic T lymphocyte-based cancer immunotherapy.
Bendle GM, Holler A, Downs AM, Xue SA, Stauss HJ. Bendle GM, et al. Expert Opin Biol Ther. 2005 Sep;5(9):1183-92. doi: 10.1517/14712598.5.9.1183. Expert Opin Biol Ther. 2005. PMID: 16120049 Review. - Immunotherapy II: Antigens, receptors and costimulation.
Searle PF, Young LS. Searle PF, et al. Cancer Metastasis Rev. 1996 Sep;15(3):329-49. doi: 10.1007/BF00046346. Cancer Metastasis Rev. 1996. PMID: 9034595 Review. - Specific immunotherapy of cancer in elderly patients.
Matzku S, Zöller M. Matzku S, et al. Drugs Aging. 2001;18(9):639-64. doi: 10.2165/00002512-200118090-00002. Drugs Aging. 2001. PMID: 11599633 Review. - Advances in personalized neoantigen vaccines for cancer immunotherapy.
Sun C, Xu S. Sun C, et al. Biosci Trends. 2020 Nov 4;14(5):349-353. doi: 10.5582/bst.2020.03267. Epub 2020 Sep 10. Biosci Trends. 2020. PMID: 32908077 Review. - Challenges and future perspectives of T cell immunotherapy in cancer.
de Aquino MT, Malhotra A, Mishra MK, Shanker A. de Aquino MT, et al. Immunol Lett. 2015 Aug;166(2):117-33. doi: 10.1016/j.imlet.2015.05.018. Epub 2015 Jun 19. Immunol Lett. 2015. PMID: 26096822 Free PMC article. Review.
Cited by
- WT1 Cancer Vaccine in Advanced Pancreatic Cancer: A Systematic Review.
Gugulothu KN, Anvesh Sai P, Suraparaju S, Karuturi SP, Pendli G, Kamma RB, Nimmagadda K, Modepalli A, Mamilla M, Vashist S. Gugulothu KN, et al. Cureus. 2024 Mar 26;16(3):e56934. doi: 10.7759/cureus.56934. eCollection 2024 Mar. Cureus. 2024. PMID: 38665761 Free PMC article. Review. - Unlocking the potential of amorphous calcium carbonate: A star ascending in the realm of biomedical application.
Liu H, Wen Z, Liu Z, Yang Y, Wang H, Xia X, Ye J, Liu Y. Liu H, et al. Acta Pharm Sin B. 2024 Feb;14(2):602-622. doi: 10.1016/j.apsb.2023.08.027. Epub 2023 Sep 1. Acta Pharm Sin B. 2024. PMID: 38322345 Free PMC article. Review. - Systemic infusion of TLR3-ligand and IFN-α in patients with breast cancer reprograms local tumor microenvironments for selective CTL influx.
Gandhi S, Opyrchal M, Grimm MJ, Slomba RT, Kokolus KM, Witkiewicz A, Attwood K, Groman A, Williams L, Tarquini ML, Wallace PK, Soh KT, Minderman H, Maguire O, O'Connor TL, Early AP, Levine EG, Kalinski P. Gandhi S, et al. J Immunother Cancer. 2023 Nov;11(11):e007381. doi: 10.1136/jitc-2023-007381. J Immunother Cancer. 2023. PMID: 37963636 Free PMC article. - Social, Genetics and Histopathological Factors Related to Titin (TTN) Gene Mutation and Survival in Women with Ovarian Serous Cystadenocarcinoma: Bioinformatics Analysis.
Gomes FC, Figueiredo ERL, Araújo EN, Andrade EM, Carneiro CDL, Almeida GM, Dias HAAL, Teixeira LIB, Almeida MT, Farias MF, Linhares NA, Fonseca NLD, Pereira YDS, Melo-Neto JS. Gomes FC, et al. Genes (Basel). 2023 May 16;14(5):1092. doi: 10.3390/genes14051092. Genes (Basel). 2023. PMID: 37239452 Free PMC article. - TFAM deficiency in dendritic cells leads to mitochondrial dysfunction and enhanced antitumor immunity through cGAS-STING pathway.
Lu T, Zhang Z, Bi Z, Lan T, Zeng H, Liu Y, Mo F, Yang J, Chen S, He X, Hong W, Zhang Z, Pi R, Ren W, Tian X, Wei Y, Luo M, Wei X. Lu T, et al. J Immunother Cancer. 2023 Mar;11(3):e005430. doi: 10.1136/jitc-2022-005430. J Immunother Cancer. 2023. PMID: 36858460 Free PMC article.
References
- Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007;70:1–11. - PubMed
- Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–33. - PubMed
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. - PubMed
- Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA088843/CA/NCI NIH HHS/United States
- U19CA72108/CA/NCI NIH HHS/United States
- U19 CA072108-06/CA/NCI NIH HHS/United States
- P50 CA062924/CA/NCI NIH HHS/United States
- CA85374/CA/NCI NIH HHS/United States
- P50CA88843/CA/NCI NIH HHS/United States
- R01 CA101190-01/CA/NCI NIH HHS/United States
- CA101190/CA/NCI NIH HHS/United States
- R01 CA085374/CA/NCI NIH HHS/United States
- R01 CA085374-01/CA/NCI NIH HHS/United States
- UL1 TR002319/TR/NCATS NIH HHS/United States
- R01 CA101190/CA/NCI NIH HHS/United States
- P50 CA062924-140014/CA/NCI NIH HHS/United States
- CA62924/CA/NCI NIH HHS/United States
- P50 CA088843-010006/CA/NCI NIH HHS/United States
- U19 CA072108/CA/NCI NIH HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources