Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions - PubMed (original) (raw)
Review
Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions
Byron Caughey et al. Annu Rev Biochem. 2009.
Abstract
The prion (infectious protein) concept has evolved with the discovery of new self-propagating protein states in organisms as diverse as mammals and fungi. The infectious agent of the mammalian transmissible spongiform encephalopathies (TSE) has long been considered the prototypical prion, and recent cell-free propagation and biophysical analyses of TSE infectivity have now firmly established its prion credentials. Other disease-associated protein aggregates, such as some amyloids, can also have prion-like characteristics under certain experimental conditions. However, most amyloids appear to lack the natural transmissibility of TSE prions. One feature that distinguishes the latter from the former is the glycophosphatidylinositol membrane anchor on prion protein, the molecule that is corrupted in TSE diseases. The presence of this anchor profoundly affects TSE pathogenesis, which involves major membrane distortions in the brain, and may be a key reason for the greater neurovirulence of TSE prions relative to many other autocatalytic protein aggregates.
Figures
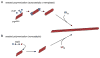
Figure 1
Seeded PrP polymerization mechanisms. In the non-catalytic model, the conformational interchange between the PrPC and PrPRES conformations is rapid, but the PrPRES conformer is poorly populated unless stabilized by binding to an existing PrPRES multimer. In the autocatalytic model, the conformational conversion of PrPC to PrPRES is rare unless catalyzed by contact with an existing PrPRES multimer.
Figure 2
Models of TSE prion amplification by PMCA. A. PMCA flow chart based on (3, 4). B. Seeded polymerization model for PrPRES formation with the aid of a polyanion (e.g. polyA RNA) (4). Adapted from (156).
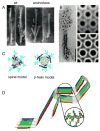
Figure 3
Ultrastructures and models of PrPRES fibrils. A. Negative-stained transmission electron micrographs of proteinase K-treated wild-type or GPI-anchorless PrPRES amyloid fibrils (34). B. Electron micrographs and crystallographically refined images of hexagonal 2D crystals and of proteinase K-treated PrPRES. On the left, the 2D crystals are shown together with fibrils. Adapted from (45). C. Top-down view of PrPRES trimers according to the spiral (47, 48) and β-helix (46) models. Glycans (aqua), α-helices (blue) and β-strands (green) are highlighted. Adapted from (48). D. Parallel, in-register β-sheet model of synthetic fibrils of human PrP residues 120-231. Adapted from (50).
Figure 4
Model of biogenesis and accumulation of PrPRES in scrapie-infected cells. As a GPI-anchored plasma membrane glycoprotein (upper right), PrPC is first synthesized in the endoplasmic reticulum (ER), processed in the Golgi apparatus, and transported to the cell surface (bottom). PrPRES, together with apparent cofactors, directly induces the conversion of GPI-anchored PrPC on the cell surface and/or in endosomes. PrPC that is released from the cell may be converted on extracellular deposits such as amyloid fibrils. Once PrPRES is made, it can accumulate on the cell surface, in intracellular vesicles (e.g. lysosomes) and aggresomes, or in extracellular deposits. Under conditions of mild proteasome inhibition, cytotoxic cytoplasmic PrP aggregates (e.g., aggresomes) can be found (157, 158). Scrapie infection alone can inhibit proteasomes, apparently due to the presence of cytoplasmic PrP oligomers (140).
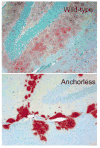
Figure 5
Different patterns of PrPRES deposition in brain tissue of scrapie-infected mice expressing wildtype (top) versus anchorless PrPC (bottom) (29). The sections shown are from the hippocampus in the vicinity of the dentate gyrus. The photos are courtesy of Dr. Bruce Chesebro, Rocky Mountain Laboratories.
Figure 6
Neuritic transport of PrPRES during acute infection. SN56 mouse neuroblastoma cells were treated with Alexa Fluor 568-labeled mouse PrPRES and imaged at 4 d post-infection. Arrows (left panel) indicate examples of fluorescent PrPRES particles transported within neuritic processes that contact other neurites and cells (89). Left panel shows Alexa Fluor 568 fluorescence. Right panel shows Alexa Fluor 568 fluorescence superimposed on a differential interference contrast image.
Figure 7
Potential mechanisms of intercellular spread of prions. A) Exosomes/membrane microparticles. Prion-infected cells release PrPRES-containing membrane vesicles that deliver PrPRES (red squares) to membranes of uninfected cells to initiate new PrPRES formation (blue squares) in recipient cells (–97). These include exosomes, vesicles generated by invagination of the limiting membranes of multivesicular bodies (MVBs) and released by MVB fusion with the plasma membrane (100). Delivery of membrane vesicles could occur at the cell surface (not shown) or in an endocytic compartment. B) Tunneling nanotubes (TNTs). TNTs are visualized as thin intercellular projections (Top view, Merged, arrow) through space (see Side view, Merged) in a co-culture of two cell lines expressing either a GPI-anchored protein tagged with GFP (green) or one tagged with mCherry (magenta) fluorescent protein. The arrowhead (Top view, Merged, white area) shows a TNT that has facilitated the transfer of GFP-tagged protein onto the surface of the mCherry-expressing cell. Side view (Merged) corresponds to a side view of a 3D volume rendering of the area indicated by the dotted rectangle in Top view (Merged). DIC, differential interference contrast image.
Figure 8
Abnormal PrP (PrPd) accumulation and membrane distortions on the surface of neurons and astrocytes in scrapie-infected sheep brain tissue. PrPd is labeled with immunogold particles. A. PrPd on the plasma membrane of a neuron without morphological changes. B. Complex dendrite membrane disturbances (microfolding-asterisks) associated with the formation of abnormal pits (not immunolabeled). C. PrPd accumulation associated with the formation of excess coated pits (arrows) and with the transfer to membranes of adjacent processes (arrow heads). D. coated membrane invaginations, one of which shows a spiral, twisted neck (not immunolabeled). E. PrPd on the plasma membrane of astrocytic processes containing abundant glial filaments. Parts of the plasmalemma of some processes have linear segments (arrowheads) suggesting increased membrane rigidity and incipient fibril formation. Adapted from (78).
Similar articles
- Prions and their partners in crime.
Caughey B, Baron GS. Caughey B, et al. Nature. 2006 Oct 19;443(7113):803-10. doi: 10.1038/nature05294. Nature. 2006. PMID: 17051207 Review. - Molecular aspects of disease pathogenesis in the transmissible spongiform encephalopathies.
Priola SA, Vorberg I. Priola SA, et al. Mol Biotechnol. 2006 May;33(1):71-88. doi: 10.1385/MB:33:1:71. Mol Biotechnol. 2006. PMID: 16691009 Review. - Amyloids, prions and the inherent infectious nature of misfolded protein aggregates.
Soto C, Estrada L, Castilla J. Soto C, et al. Trends Biochem Sci. 2006 Mar;31(3):150-5. doi: 10.1016/j.tibs.2006.01.002. Epub 2006 Feb 13. Trends Biochem Sci. 2006. PMID: 16473510 Review. - Prions, prionoid complexes and amyloids: the bad, the good and something in between.
Hafner Bratkovič I. Hafner Bratkovič I. Swiss Med Wkly. 2017 Apr 18;147:w14424. doi: 10.4414/smw.2017.14424. eCollection 2017. Swiss Med Wkly. 2017. PMID: 28421568 Review. - Reconstructing prions: fibril assembly from simple yeast to complex mammals.
Sigurdson C, Polymenidou M, Aguzzi A. Sigurdson C, et al. Neurodegener Dis. 2005;2(1):1-5. doi: 10.1159/000086425. Neurodegener Dis. 2005. PMID: 16908997 Review.
Cited by
- Tau mutant A152T, a risk factor for FTD/PSP, induces neuronal dysfunction and reduced lifespan independently of aggregation in a C. elegans Tauopathy model.
Pir GJ, Choudhary B, Mandelkow E, Mandelkow EM. Pir GJ, et al. Mol Neurodegener. 2016 Apr 27;11:33. doi: 10.1186/s13024-016-0096-1. Mol Neurodegener. 2016. PMID: 27118310 Free PMC article. - Comparative profiling of highly enriched 22L and Chandler mouse scrapie prion protein preparations.
Moore RA, Timmes A, Wilmarth PA, Priola SA. Moore RA, et al. Proteomics. 2010 Aug;10(15):2858-69. doi: 10.1002/pmic.201000104. Proteomics. 2010. PMID: 20518029 Free PMC article. - Understanding Prion Strains: Evidence from Studies of the Disease Forms Affecting Humans.
Rossi M, Baiardi S, Parchi P. Rossi M, et al. Viruses. 2019 Mar 29;11(4):309. doi: 10.3390/v11040309. Viruses. 2019. PMID: 30934971 Free PMC article. Review. - Detection of Aggregation-Competent Tau in Neuron-Derived Extracellular Vesicles.
Guix FX, Corbett GT, Cha DJ, Mustapic M, Liu W, Mengel D, Chen Z, Aikawa E, Young-Pearse T, Kapogiannis D, Selkoe DJ, Walsh DM. Guix FX, et al. Int J Mol Sci. 2018 Feb 27;19(3):663. doi: 10.3390/ijms19030663. Int J Mol Sci. 2018. PMID: 29495441 Free PMC article. - Transthyretin Stabilizers and Seeding Inhibitors as Therapies for Amyloid Transthyretin Cardiomyopathy.
Morfino P, Aimo A, Vergaro G, Sanguinetti C, Castiglione V, Franzini M, Perrone MA, Emdin M. Morfino P, et al. Pharmaceutics. 2023 Apr 3;15(4):1129. doi: 10.3390/pharmaceutics15041129. Pharmaceutics. 2023. PMID: 37111614 Free PMC article. Review.
References
- Griffith JS. Self-replication and scrapie. Nature. 1967;215:1043–4. - PubMed
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–44. - PubMed
- Castilla J, Saa P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. - PubMed
- Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae [see comments] Science. 1994;264:566–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources