Molecular targeting of MLL-rearranged leukemia cell lines with the synthetic peptide PFWT synergistically enhances the cytotoxic effect of established chemotherapeutic agents - PubMed (original) (raw)
Molecular targeting of MLL-rearranged leukemia cell lines with the synthetic peptide PFWT synergistically enhances the cytotoxic effect of established chemotherapeutic agents
Cecily A Bennett et al. Leuk Res. 2009 Jul.
Abstract
MLL leukemias are characterized cytogenetically by reciprocal translocations of the MLL gene at 11q23 and clinically by unfavorable outcomes. Evidence indicating that MLL leukemias are resistant to apoptosis encourages the identification of agents that induce cell death by other mechanisms. The AF4-mimetic peptide PFWT induces necrosis in the t(4;11) leukemia cell line, MV4-11. Treatment of MV4-11 cells with PFWT in combination with four chemotherapeutic compounds results in sequence-dependent synergy, induction of both apoptotic and necrotic cell death, and inhibition of MV4-11 clonogenicity. Therefore, PFWT holds promise as a therapy for MLL leukemias that augments the effects of several clinically available chemotherapeutic agents.
Figures
Figure 1
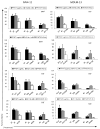
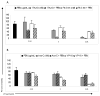
Treatment of MV4-11 and MOLM-13 cells with PFWT in combination with chemotherapeutic drugs results in an overall decrease in cell viability compared to treatment with PFWT or chemotherapeutic drug alone. MV4-11 cells were treated simultaneously (SIMT) with PFWT (μg/mL) and either 17AAG (nM) or a Flt-3 Inhibitor (nM) for 72 h or sequentially (SQT) with PFWT (μg/mL) and either cytarabine (nM) or etoposide (nM) for 72 h. In the latter case (SQT), cells were pretreated with either cytarabine or etoposide for 24 h prior to the addition of PFWT. MOLM-13 cells were treated sequentially with PFWT (μg/mL) and either 17AAG (nM), cytarabine (nM), a Flt3 inhibitor (nM) or etoposide (nM) for 72 h. For combined drug treatment of MOLM-13 cells, cells were pre-treated with individual drugs 24 h prior to the addition of PFWT. Data is a combination of at least three experiments and standard error of the mean (SEM) is represented by error bars. AraC = cytarabine; VP-16 = etoposide.
Figure 2
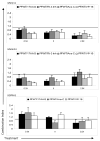
Combination indices (CI) for drug interactions were calculated from cytotoxicity assays. CI values are plotted as a function of drug concentration for each of the respective cell lines and drug combinations. CI>1: antagonistic; CI=1: additive; CI<1: synergistic. Drugs were administered in fixed dose combinations where X = IC50 for each drug. AraC = cytarabine; VP-16 = etoposide. The CI values were generated using Calcusyn software as described in Materials and Methods.
Figure 3
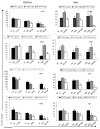
Treatment of KOPN-8 and RAJI cells with PFWT in combination with chemotherapeutic drugs induces a modest decrease in cell viability compared to treatment with PFWT or drugs alone. KOPN-8 cells were treated simultaneously (SIMT) with PFWT (μg/mL) and either 17AAG (nM), a Flt-3 Inhibitor (nM), cytarabine (nM) or etoposide (nM) for 72 h. RAJI cells were treated simultaneously (SIMT) with PFWT (μg/mL) and either 17AAG (nM) or a Flt-3 Inhibitor (nM) for 72 h. They were treated sequentially (SEQ) with PFWT (μg/mL) and either cytarabine (nM) or etoposide (nM) for 72 h. In sequentially treated cells (SEQ), cells were pretreated with either cytarabine or etoposide for 24 h followed by the addition of PFWT. Standard error of the mean (SEM) is represented by error bars. AraC = cytarabine; VP-16 = etoposide.
Figure 4
Treatment of IMCD3 cells with PFWT in combination with chemotherapeutic drugs induces a modest decrease in cell viability compared to treatment with PFWT or drugs alone. (A) IMCD3 cells were treated simultaneously (SIMT) with PFWT (μg/mL) and either cytarabine (nM) or etoposide (nM) for 72 h. Standard error of the mean (SEM) is represented by error bars. AraC = cytarabine; VP-16 = etoposide. (B) CI values are plotted as a function of drug concentration and drug combinations. CI>1: antagonistic; CI=1: additive; CI<1: synergistic. Drugs were administered in fixed dose combinations where X = IC50 for each drug. AraC = cytarabine; VP-16 = etoposide. The CI values were generated using Calcusyn software as described in Materials and Methods.
Figure 5
Treatment of MV4-11 with penetratin transport sequence in combination with chemotherapeutic drugs did not contribute to drug cytotoxicity. MV4-11 cells were treated simultaneously (SIMT) with penetratin (μg/mL) and either 17AAG (nM) or a Flt-3 Inhibitor (nM) for 72 h or sequentially (SQT) with penetratin (μg/mL) and either cytarabine (nM) or etoposide (nM) for 72 h. In the latter case (SQT), cells were pretreated with either cytarabine or etoposide for 24 h prior to the addition of PFWT. Data is a combination of at least three experiments and standard error of the mean (SEM) is represented by error bars. AraC = cytarabine; VP-16 = etoposide.
Figure 6
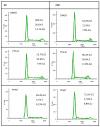
PFWT treatment causes accumulation of MV4-11 cells in the G0/G1 phase of the cell cycle. MV4-11 cells were treated for 6h (a) or 24h (b) with 25 μg/mL PFWT, PFmut, or equivalent volume DMSO. Percentages of the singlet population in each phase of the cell cycle are shown as insets for each graph. Note that the y-axis scales for PFWT-treated samples are different from those of control samples, due to fewer events in the live singlet gates.
Figure 7
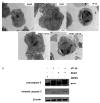
MV4-11 cells undergo both necrotic and apoptotic cell death after co-treatment with PFWT and etoposide. (A) MV4-11 cells were treated at the IC50 for PFWT and etoposide alone and in combination for a total of 48 h. Ultrastructural changes were observed by transmission electron microscopy. Note nuclear condensation (apoptosis) and membrane disintegration (necrosis) occurring simultaneously in cells treated with PFWT and etoposide in combination. Magnification is 2950x and bars represent 2 μm. (B) MV4-11 cells were treated with VP-16 and PFWT alone or in combination for a total of 48h, at dosages corresponding to the respective IC50 values for both agents. Western blotting for caspase-3 was performed on whole cell lysates of treated cells, using an antibody capable of detecting both the proenzyme (35 kDa, arrow) and active caspase-3 (12 kDa). Beta-actin was probed as a loading control.
Figure 8
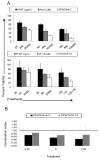
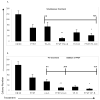
Clonogenic proliferation of MV4-11 cells is inhibited by combined treatment with PFWT and 17AAG or cytarabine. MV4-11 cells were treated with PFWT and chemotherapeutic agents alone and in combination. For these experiments, the in vitro IC50 for each individual compound was used. After 72h drug exposure, cells were plated in methylcellulose medium and incubated for 10 days, as described in Section 2.7. (A) PFWT (μg/mL) and 17AAG (nM) or Flt-3 inhibitor (nM) or (B) PFWT (μg/mL) and cytarabine (Ara-C) (nM) or etoposide (VP-16) (nM). Schematics of the sequence of treatment are shown as inlays in the upper right hand corner of graphs. One asterisk (*) indicates decreased colony number relative to PFWT alone; two asterisks (**) indicates decreased colony number relative to both PFWT and chemotherapeutic agent alone (p < 0.05). Significance was determined using Student’s t-test with two sample unequal variance and one-tailed distribution.
Similar articles
- The AF4-mimetic peptide, PFWT, induces necrotic cell death in MV4-11 leukemia cells.
Palermo CM, Bennett CA, Winters AC, Hemenway CS. Palermo CM, et al. Leuk Res. 2008 Apr;32(4):633-42. doi: 10.1016/j.leukres.2007.08.002. Epub 2007 Sep 17. Leuk Res. 2008. PMID: 17875318 Free PMC article. - t(4;11) leukemias display addiction to MLL-AF4 but not to AF4-MLL.
Kumar AR, Yao Q, Li Q, Sam TA, Kersey JH. Kumar AR, et al. Leuk Res. 2011 Mar;35(3):305-9. doi: 10.1016/j.leukres.2010.08.011. Epub 2010 Sep 25. Leuk Res. 2011. PMID: 20869771 Free PMC article. - The synthetic peptide PFWT disrupts AF4-AF9 protein complexes and induces apoptosis in t(4;11) leukemia cells.
Srinivasan RS, Nesbit JB, Marrero L, Erfurth F, LaRussa VF, Hemenway CS. Srinivasan RS, et al. Leukemia. 2004 Aug;18(8):1364-72. doi: 10.1038/sj.leu.2403415. Leukemia. 2004. PMID: 15269783 - _MLL_-Rearranged Acute Leukemia with t(4;11)(q21;q23)-Current Treatment Options. Is There a Role for CAR-T Cell Therapy?
Britten O, Ragusa D, Tosi S, Kamel YM. Britten O, et al. Cells. 2019 Oct 29;8(11):1341. doi: 10.3390/cells8111341. Cells. 2019. PMID: 31671855 Free PMC article. Review. - Malignant hematopoietic cell lines: in vitro models for the study of MLL gene alterations.
Drexler HG, Quentmeier H, MacLeod RA. Drexler HG, et al. Leukemia. 2004 Feb;18(2):227-32. doi: 10.1038/sj.leu.2403236. Leukemia. 2004. PMID: 14671638 Review.
Cited by
- Binding cavities and druggability of intrinsically disordered proteins.
Zhang Y, Cao H, Liu Z. Zhang Y, et al. Protein Sci. 2015 May;24(5):688-705. doi: 10.1002/pro.2641. Epub 2015 Feb 24. Protein Sci. 2015. PMID: 25611056 Free PMC article. - An AF9/ENL-targted peptide with therapeutic potential in mixed lineage leukemias.
Barretto NN, Karahalios DS, You D, Hemenway CS. Barretto NN, et al. J Exp Ther Oncol. 2014;10(4):293-300. J Exp Ther Oncol. 2014. PMID: 25509985 Free PMC article. - The molecular biology of mixed lineage leukemia.
Slany RK. Slany RK. Haematologica. 2009 Jul;94(7):984-93. doi: 10.3324/haematol.2008.002436. Epub 2009 Jun 16. Haematologica. 2009. PMID: 19535349 Free PMC article. Review. - Molecular and Epigenetic Mechanisms of MLL in Human Leukemogenesis.
Ballabio E, Milne TA. Ballabio E, et al. Cancers (Basel). 2012 Sep 10;4(3):904-44. doi: 10.3390/cancers4030904. Cancers (Basel). 2012. PMID: 24213472 Free PMC article. - Development of a high-throughput screening-compatible assay for the discovery of inhibitors of the AF4-AF9 interaction using AlphaScreen technology.
Watson VG, Drake KM, Peng Y, Napper AD. Watson VG, et al. Assay Drug Dev Technol. 2013 May;11(4):253-68. doi: 10.1089/adt.2012.495. Assay Drug Dev Technol. 2013. PMID: 23679849 Free PMC article.
References
- Daser A, Rabbitts TH. Extending the repertoire of the mixed-lineage leukemia gene MLL in leukemogenesis. Genes Dev. 2004;18(9):965–974. - PubMed
- Biondi A, Cimino G, Pieters R, Pui CH. Biological and therapeutic aspects of infant leukemia. Blood. 2000;96(1):24–33. - PubMed
- Pui CH, Gaynon PS, Boyett JM, Chessells JM, Baruchel A, Kamps W, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359(9321):1909–1915. - PubMed
- Nakamura T, Mori T, Tada S, Krajewski W, Rozovskaia T, Wassell R, et al. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol Cell. 2002;10(5):1119–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR020152-047685/RR/NCRR NIH HHS/United States
- R01 CA098459-01A2/CA/NCI NIH HHS/United States
- P20 RR020152-010002/RR/NCRR NIH HHS/United States
- R01 CA098459-03/CA/NCI NIH HHS/United States
- CA 098459/CA/NCI NIH HHS/United States
- R01 CA098459/CA/NCI NIH HHS/United States
- P20 RR020152-020002/RR/NCRR NIH HHS/United States
- P20 RR020152-037527/RR/NCRR NIH HHS/United States
- RR 020152/RR/NCRR NIH HHS/United States
- R01 CA098459-02/CA/NCI NIH HHS/United States
- P20 RR020152-056902/RR/NCRR NIH HHS/United States
- P20 RR020152/RR/NCRR NIH HHS/United States
- R01 CA098459-03S1/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical