The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells - PubMed (original) (raw)
The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells
Seth B Coffelt et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Bone marrow-derived mesenchymal stem cells or multipotent mesenchymal stromal cells (MSCs) have been shown to engraft into the stroma of several tumor types, where they contribute to tumor progression and metastasis. However, the chemotactic signals mediating MSC migration to tumors remain poorly understood. Previous studies have shown that LL-37 (leucine, leucine-37), the C-terminal peptide of human cationic antimicrobial protein 18, stimulates the migration of various cell types and is overexpressed in ovarian, breast, and lung cancers. Although there is evidence to support a pro-tumorigenic role for LL-37, the function of the peptide in tumors remains unclear. Here, we demonstrate that neutralization of LL-37 in vivo significantly reduces the engraftment of MSCs into ovarian tumor xenografts, resulting in inhibition of tumor growth as well as disruption of the fibrovascular network. Migration and invasion experiments conducted in vitro indicated that the LL-37-mediated migration of MSCs to tumors likely occurs through formyl peptide receptor like-1. To assess the response of MSCs to the LL-37-rich tumor microenvironment, conditioned medium from LL-37-treated MSCs was assessed and found to contain increased levels of several cytokines and pro-angiogenic factors compared with controls, including IL-1 receptor antagonist, IL-6, IL-10, CCL5, VEGF, and matrix metalloproteinase-2. Similarly, Matrigel mixed with LL-37, MSCs, or the combination of the two resulted in a significant number of vascular channels in nude mice. These data indicate that LL-37 facilitates ovarian tumor progression through recruitment of progenitor cell populations to serve as pro-angiogenic factor-expressing tumor stromal cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
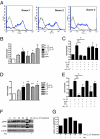
LL-37 mediates MSC migration and invasion through a G protein-coupled receptor. (A) FPRL1 expression on 3 different donor pools of MSCs analyzed by flow cytometry. (B) Graphic representation of MSC migration stimulated as indicated in a modified Boyden chamber. EGF and PMA were used at 10 ng/mL. (C) MSC migration after pretreatment of cells with 100 ng/mL pertussis toxin (Ptx), or preincubation of LL-37 and EGF with an anti-LL-37 neutralizing antibody (αLL-37). (D) Invasion of MSCs through Matrigel-coated inserts following stimulation as indicated. (E) MSC invasion after pretreatment of cells with Ptx or preincubation of LL-37 and EGF with αLL-37 antibody. *, P < 0.05; **, P < 0.01. (F) Lysates from LL-37-treated MSCs analyzed for ERK phosphorylation by Western blot. MSCs in the far right lane were pretreated with Ptx for 1 h before stimulation with LL-37 for 10 min. M = molecular weight marker. (G) Quantification of Western blot band intensity by densitometry (n = 3), plotted as a bar graph.
Fig. 2.
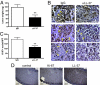
Inhibition of LL-37 significantly reduces engraftment of MSCs into ovarian tumors. Human ovarian tumor xenografts were established i.p. in SCID mice. Mice were treated with IgG or anti-LL-37 antibodies (αLL-37) twice a week for 4 weeks. ffLUC-labeled MSCs were injected 4 times at weekly intervals 1 day after the first weekly injection of antibody, then visualized by bioluminescence in live mice. (A) Representative images of MSC engraftment into ovarian tumors 7 days after each injection of MSC. (B) Quantification of luminescence units emanating from tumor-engrafted MSCs. Values are mean ± SE. *P < 0.05. (C) Expression of LL-37 (red) in ovarian tumor sections of IgG- and αLL-37-treated mice. MSCs were identified using an anti-ffLUC antibody (green) and are indicated by white arrows. Nuclei were detected with DAPI. Sections are magnified ×100. (Scale bar, 100 μm.) (D) High-powered image (×400) of tumor section fluorescently labeled as described. (E) Sequential section of D immunohistochemically stained with anti-LL-37 antibodies followed by hematoxylin counterstain. (F) Example of LL-37-expressing MSCs in perivascular areas. (Scale bar, 50 μm, D–F.)
Fig. 3.
Growth of ovarian tumor xenografts is diminished by neutralization of LL-37. (A) Graphic representation of tumor weights from IgG- (n = 10) and αLL-37-treated (n = 9) animals obtained after surgical removal. Values are mean ± SE. **, P < 0.01. (B) Representative images of tumors stained for Ki-67 with hematoxylin counterstain. Arrows indicate mouse stroma in human xenograft tumors. (Scale bar, 50 μm.) (C) Graphic representation of the average number of Ki-67+ nuclei per high-powered field. Values are mean ± SE. **, P < 0.01. (D) Expression of Ki-67 and LL-37 in tumor necrotic region from an αLL-37-treated mouse. (Scale bar, 100 μm.)
Fig. 4.
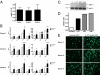
MSCs secrete increased levels of angiogenic and inflammatory mediators after LL-37 stimulation. (A) The concentration of LL-37 in conditioned medium taken from unstimulated MSCs in culture. (B) Serum-starved MSCs were treated with 5 μg/mL LL-37 for 48 h, then conditioned medium was analyzed by Luminex-based cytokine arrays. Values are mean ± SE. *, P < 0.05, **, P < 0.01. (C) Analysis of MSC-conditioned medium after treatment for 48 h as indicated by gelatin zymography. The representative image depicts the electrophorectic pro-MMP-2 (72 kDa) and active MMP-2 (62 kDa). MMP-9 (92 kDa) was not undetectable. (D) Quantification of zymography by densitometry. Intensity of the lower band (active MMP-2, 62 kDa) is plotted as a bar graph (n = 3). (E) Conditioned medium from untreated and LL-37-treated MSCs was added to HUVECs, then seeded onto Matrigel. Fluorescently labeled cells were monitored were for formation of capillary-like tubules. Photographs are representative of HUVECs after 2 h incubation.
Fig. 5.
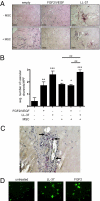
LL-37 enhances the pro-angiogenic activity of MSCs. (A) LL-37 or the combination of FGF and VEGFA was added to cold Matrigel with or without MSCs and injected into nude mice (n < 6). The absence of growth factors and cells served as negative control. After 7 to 10 days, Matrigel plugs were surgically removed, fixed, sectioned, and stained by H&E. Representative images of vascular channels are shown. (Scale bar, 100 μm.) (B) The average number of vascular channels in each plug section was determined by counting 3 high-powered fields of view then graphically represented. Values are mean ± SE. *, P < 0.05, **, P < 0.01, ***, P < 0.001. (C) Example of MSCs in perivascular areas identified by Ki-67 staining. (D) Fluorescently labeled, serum-starved MSCs were seeded onto Matrigel in the presence of 5 μg/mL LL-37 or 10 ng/mL FGF2 and allowed to incubate overnight. Formation of tubules, indicative of their pericyte-like differentiation, was captured by microscopy at ×200 the next day.
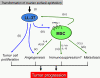
Fig. 6.
Schematic illustration of effects of LL-37 and MSCs on ovarian tumor progression (see Discussion).
Similar articles
- Leucine leucine-37 uses formyl peptide receptor-like 1 to activate signal transduction pathways, stimulate oncogenic gene expression, and enhance the invasiveness of ovarian cancer cells.
Coffelt SB, Tomchuck SL, Zwezdaryk KJ, Danka ES, Scandurro AB. Coffelt SB, et al. Mol Cancer Res. 2009 Jun;7(6):907-15. doi: 10.1158/1541-7786.MCR-08-0326. Epub 2009 Jun 2. Mol Cancer Res. 2009. PMID: 19491199 Free PMC article. - LL-37 stimulates the functions of adipose-derived stromal/stem cells via early growth response 1 and the MAPK pathway.
Yang Y, Choi H, Seon M, Cho D, Bang SI. Yang Y, et al. Stem Cell Res Ther. 2016 Apr 19;7(1):58. doi: 10.1186/s13287-016-0313-4. Stem Cell Res Ther. 2016. PMID: 27095351 Free PMC article. - Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion.
Coffelt SB, Waterman RS, Florez L, Höner zu Bentrup K, Zwezdaryk KJ, Tomchuck SL, LaMarca HL, Danka ES, Morris CA, Scandurro AB. Coffelt SB, et al. Int J Cancer. 2008 Mar 1;122(5):1030-9. doi: 10.1002/ijc.23186. Int J Cancer. 2008. PMID: 17960624 - The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: Pro-Tumorigenic Effects versus Therapeutic Potential.
Slama Y, Ah-Pine F, Khettab M, Arcambal A, Begue M, Dutheil F, Gasque P. Slama Y, et al. Int J Mol Sci. 2023 Aug 31;24(17):13511. doi: 10.3390/ijms241713511. Int J Mol Sci. 2023. PMID: 37686315 Free PMC article. Review. - Tumor Microenvironment Uses a Reversible Reprogramming of Mesenchymal Stromal Cells to Mediate Pro-tumorigenic Effects.
Nwabo Kamdje AH, Seke Etet PF, Simo Tagne R, Vecchio L, Lukong KE, Krampera M. Nwabo Kamdje AH, et al. Front Cell Dev Biol. 2020 Nov 19;8:545126. doi: 10.3389/fcell.2020.545126. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33330442 Free PMC article. Review.
Cited by
- The bone marrow microenvironment as niche retreats for hematopoietic and leukemic stem cells.
Nwajei F, Konopleva M. Nwajei F, et al. Adv Hematol. 2013;2013:953982. doi: 10.1155/2013/953982. Epub 2013 Jan 10. Adv Hematol. 2013. PMID: 23365579 Free PMC article. - Mesenchymal cell interaction with ovarian cancer cells triggers pro-metastatic properties.
Lis R, Touboul C, Raynaud CM, Malek JA, Suhre K, Mirshahi M, Rafii A. Lis R, et al. PLoS One. 2012;7(5):e38340. doi: 10.1371/journal.pone.0038340. Epub 2012 May 30. PLoS One. 2012. PMID: 22666502 Free PMC article. - Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: current status and potential future applications.
Wnorowska U, Fiedoruk K, Piktel E, Prasad SV, Sulik M, Janion M, Daniluk T, Savage PB, Bucki R. Wnorowska U, et al. J Nanobiotechnology. 2020 Jan 2;18(1):3. doi: 10.1186/s12951-019-0566-z. J Nanobiotechnology. 2020. PMID: 31898542 Free PMC article. Review. - Overview of signal transduction between LL37 and bone marrow-derived MSCs.
Zhu Y, Lu F, Zhang G, Liu Z. Zhu Y, et al. J Mol Histol. 2022 Apr;53(2):149-157. doi: 10.1007/s10735-021-10048-4. Epub 2022 Jan 20. J Mol Histol. 2022. PMID: 35048213 Review. - Leucine leucine-37 uses formyl peptide receptor-like 1 to activate signal transduction pathways, stimulate oncogenic gene expression, and enhance the invasiveness of ovarian cancer cells.
Coffelt SB, Tomchuck SL, Zwezdaryk KJ, Danka ES, Scandurro AB. Coffelt SB, et al. Mol Cancer Res. 2009 Jun;7(6):907-15. doi: 10.1158/1541-7786.MCR-08-0326. Epub 2009 Jun 2. Mol Cancer Res. 2009. PMID: 19491199 Free PMC article.
References
- Coffelt SB, et al. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int J Cancer. 2008;122:1030–1039. - PubMed
- Heilborn JD, et al. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer. 2005;114:713–719. - PubMed
- von Haussen J, et al. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59:12–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR020152-020005/RR/NCRR NIH HHS/United States
- CA-1094551/CA/NCI NIH HHS/United States
- CA49639/CA/NCI NIH HHS/United States
- P20 RR020152/RR/NCRR NIH HHS/United States
- R01 CA109451/CA/NCI NIH HHS/United States
- RC1 CA146381/CA/NCI NIH HHS/United States
- P50 CA116199/CA/NCI NIH HHS/United States
- P20 RR020152-037530/RR/NCRR NIH HHS/United States
- 1P20RR20152-01/RR/NCRR NIH HHS/United States
- CA-116199/CA/NCI NIH HHS/United States
- P20 RR020152-056904/RR/NCRR NIH HHS/United States
- P20 RR020152-010005/RR/NCRR NIH HHS/United States
- P20 RR020152-047687/RR/NCRR NIH HHS/United States
- P01 CA049639/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases