Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology - PubMed (original) (raw)
Comparative Study
Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology
Nurit Ballas et al. Nat Neurosci. 2009 Mar.
Abstract
The neurodevelopmental disorder Rett syndrome (RTT) is caused by sporadic mutations in the transcriptional factor methyl-CpG-binding protein 2 (MeCP2). Although it is thought that the primary cause of RTT is cell autonomous, resulting from a lack of functional MeCP2 in neurons, whether non-cell autonomous factors contribute to the disease is unknown. We found that the loss of MeCP2 occurs not only in neurons but also in glial cells of RTT brains. Using an in vitro co-culture system, we found that mutant astrocytes from a RTT mouse model, and their conditioned medium, failed to support normal dendritic morphology of either wild-type or mutant hippocampal neurons. Our studies suggest that astrocytes in the RTT brain carrying MeCP2 mutations have a non-cell autonomous effect on neuronal properties, probably as a result of aberrant secretion of soluble factor(s).
Figures
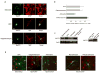
Figure 1. MeCP2 is present in all glial cell types in normal rat and mouse brains
(a) Immunostaining showing MeCP2 is present in nuclei of cultured rat glia. MeCP2 protein (green) and cell-specific marker proteins (red) are indicated. Calibration bar, 20 μm. (b) Real time RT-PCR analysis showing MeCP2 mRNA levels in rat glia. MeCP2 transcripts in cortical neurons are shown for comparison. Error bars represent standard deviation (SD) based on three independent experiments. (c) Western blot analysis showing MeCP2 protein in rat glia (left panel) and optic nerve (right panel). MeCP2 and Sin3A migrate at 75 kDa and 150kDa, respectively. (d) Co-immunostaining of rat or mouse brain sections for MeCP2 (red) and the glial-specific markers (green) as indicated. Calibration bars, 20 μm.
Figure 2. MeCP2 is detected in astrocytes in brain sections from wild-type but not MeCP2-null mice
(a) Co-immunostaining of brain sections from 6-week-old wild-type (+/y) and RTT (−/y) mice for MeCP2 (red) and GFAP (green). Arrows indicate the presence and absence of MeCP2 in astrocyte nuclei of wild-type (WT) and RTT brains, respectively. Calibration bars, 40 μm. (b) Western blot analysis confirms the presence of MeCP2 in WT and its absence in MeCP2-null astrocytes. Arrow indicates MeCP2 C-terminal peptide product of the recombination event in the Jaenisch mouse model. Sin3A serves as loading control. (c) Western blot showing an altered global chromatin signature in astrocytes from RTT mice (Mut). Histones were probed with the indicated antibodies to histone modifications.
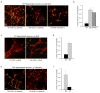
Figure 3. Wild-type hippocampal neurons co-cultured with cortical astrocytes from RTT mice exhibit stunted dendrites
(a) Aberrant morphology of hippocampal neurons, visualized by MAP staining (red), increases with time in culture. Wild-type (WT) hippocampal neurons co-cultured with either WT astrocytes (top panels) or Mutant (Mut) astrocytes (bottom panels). Note the decrease in fine processes and their shorter length of processes when co-cultured with mutant astrocytes. Calibration bar, 100 μm. (b) Immunostaining for nuclear MeCP2 (green) and MAP2 (red) showing the aberrant cytoplasmic MAP distribution (arrows) in WT hippocampal neurons cultured with Mut astrocytes. Calibration bar, 40 μm. DIV, Days In Vitro. (c) Bar graphs represent the fraction of neurons with at least two short (<50 μm) dendrites when co-cultured with WT or Mut astrocytes. Error bars represent SD based on three independent experiments.
Figure 4. Conditioned medium from MeCP2-null astrocytes cannot support normal neuronal growth
Co-immunostaining of hippocampal neurons with MAP2 as dendritic marker (red) and MeCP2 (green). (a) Hippocampal neurons from WT mice cultured for 6 days in astrocytic conditioned media (ACM) from wild-type (WT), MeCP2-null astrocytes (Mut), or with mixed ACM from WT and mutant astrocytes. (b) Bar graphs represent the fraction of neurons with at least two short (>50 mm) dendrites when cultured in the different ACM. Error bars represent SD based on three independent experiments. (c) WT hippocampal neurons cultured for 7 days with conditioned medium generated from MeCP2-null astrocytes of the Bird mouse model show similar abnormal morphology. (d) Bar graphs as in b. (e) Hippocampal neurons from RTT mice (Mut) cultured for 6 days are supported by conditioned medium from WT astrocytes (WT ACM). (f) Bar graphs as in b. Note that the gain of image in Mut hippocampal neurons is increased for MeCP2 because MeCP2-null neurons from the Jaenisch mouse model express low levels of the C-terminus, which is recognized by the anti-MeCP2 antibody used. Calibration bars, 40 μm.
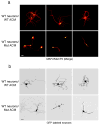
Figure 5. Altered morphology of wild-type neurons cultured with ACM from MeCP2-null astrocytes is evident at the single cell level
(a) Co-immunostaining in low-density neuronal cultures (6 DIV) with MAP2 (red) and MeCP2 (green) demonstrating aberrant process morphology when cultured in ACM from mutant astrocytes (compare top panels, WT ACM to lower panels Mut ACM). Calibration bar, 30 μm. (b) GFP-expressing neurons show aberrant processes when cultured in mutant ACM (compare top panels, WT ACM to lower panels Mut ACM). Calibration bars, 20 μm.
Similar articles
- Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate.
Maezawa I, Jin LW. Maezawa I, et al. J Neurosci. 2010 Apr 14;30(15):5346-56. doi: 10.1523/JNEUROSCI.5966-09.2010. J Neurosci. 2010. PMID: 20392956 Free PMC article. - A role for glia in the progression of Rett's syndrome.
Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, Mandel G. Lioy DT, et al. Nature. 2011 Jun 29;475(7357):497-500. doi: 10.1038/nature10214. Nature. 2011. PMID: 21716289 Free PMC article. - Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions.
Maezawa I, Swanberg S, Harvey D, LaSalle JM, Jin LW. Maezawa I, et al. J Neurosci. 2009 Apr 22;29(16):5051-61. doi: 10.1523/JNEUROSCI.0324-09.2009. J Neurosci. 2009. PMID: 19386901 Free PMC article. - Deciphering Rett syndrome with mouse genetics, epigenomics, and human neurons.
Tao J, Wu H, Sun YE. Tao J, et al. Int Rev Neurobiol. 2009;89:147-60. doi: 10.1016/S0074-7742(09)89007-7. Int Rev Neurobiol. 2009. PMID: 19900619 Review. - Glial Dysfunction in MeCP2 Deficiency Models: Implications for Rett Syndrome.
Kahanovitch U, Patterson KC, Hernandez R, Olsen ML. Kahanovitch U, et al. Int J Mol Sci. 2019 Aug 5;20(15):3813. doi: 10.3390/ijms20153813. Int J Mol Sci. 2019. PMID: 31387202 Free PMC article. Review.
Cited by
- Neural precursor cells rescue symptoms of Rett syndrome by activation of the Interferon γ pathway.
Frasca A, Miramondi F, Butti E, Indrigo M, Balbontin Arenas M, Postogna FM, Piffer A, Bedogni F, Pizzamiglio L, Cambria C, Borello U, Antonucci F, Martino G, Landsberger N. Frasca A, et al. EMBO Mol Med. 2024 Dec;16(12):3218-3246. doi: 10.1038/s44321-024-00144-9. Epub 2024 Sep 20. EMBO Mol Med. 2024. PMID: 39304759 Free PMC article. - Methyl-CpG Binding Protein 2 Regulates Microglia and Macrophage Gene Expression in Response to Inflammatory Stimuli.
Cronk JC, Derecki NC, Ji E, Xu Y, Lampano AE, Smirnov I, Baker W, Norris GT, Marin I, Coddington N, Wolf Y, Turner SD, Aderem A, Klibanov AL, Harris TH, Jung S, Litvak V, Kipnis J. Cronk JC, et al. Immunity. 2015 Apr 21;42(4):679-91. doi: 10.1016/j.immuni.2015.03.013. Immunity. 2015. PMID: 25902482 Free PMC article. - Modeling disrupted synapse formation in wolfram syndrome using hESCs-derived neural cells and cerebral organoids identifies Riluzole as a therapeutic molecule.
Yuan F, Li Y, Hu R, Gong M, Chai M, Ma X, Cha J, Guo P, Yang K, Li M, Xu M, Ma Q, Su Q, Zhang C, Sheng Z, Wu H, Wang Y, Yuan W, Bian S, Shao L, Zhang R, Li K, Shao Z, Zhang ZN, Li W. Yuan F, et al. Mol Psychiatry. 2023 Apr;28(4):1557-1570. doi: 10.1038/s41380-023-01987-3. Epub 2023 Feb 7. Mol Psychiatry. 2023. PMID: 36750736 Free PMC article. - Autism susceptibility candidate 2 (Auts2) encodes a nuclear protein expressed in developing brain regions implicated in autism neuropathology.
Bedogni F, Hodge RD, Nelson BR, Frederick EA, Shiba N, Daza RA, Hevner RF. Bedogni F, et al. Gene Expr Patterns. 2010 Jan;10(1):9-15. doi: 10.1016/j.gep.2009.11.005. Epub 2009 Dec 3. Gene Expr Patterns. 2010. PMID: 19948250 Free PMC article. - Child and adolescent psychiatric genetics.
Hebebrand J, Scherag A, Schimmelmann BG, Hinney A. Hebebrand J, et al. Eur Child Adolesc Psychiatry. 2010 Mar;19(3):259-79. doi: 10.1007/s00787-010-0091-y. Epub 2010 Feb 6. Eur Child Adolesc Psychiatry. 2010. PMID: 20140632 Review.
References
- Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8. - PubMed
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–37. - PubMed
- Chandler SP, Guschin D, Landsberger N, Wolffe AP. The methyl-CpG binding transcriptional repressor MeCP2 stably associates with nucleosomal DNA. Biochemistry. 1999;38:7008–18. - PubMed
- Kriaucionis S, Bird A. DNA methylation and Rett syndrome. Hum Mol Genet. 2003;12(Spec No 2):R221–7. - PubMed
- Harikrishnan KN, et al. Brahma links the SWI/SNF chromatin-remodeling complex with MeCP2-dependent transcriptional silencing. Nat Genet. 2005;37:254–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases