An inverse association of cardiovascular risk and frontal lobe glucose metabolism - PubMed (original) (raw)
An inverse association of cardiovascular risk and frontal lobe glucose metabolism
B Kuczynski et al. Neurology. 2009.
Abstract
Objective: To investigate associations between vascular risk profile and cerebral glucose metabolism.
Methods: Subjects ranged from normal to having dementia (age >55 years) and underwent neuropsychological testing, MRI, and FDG PET scanning (n = 58). The Framingham Cardiovascular Risk Profile (FCRP) and its individual components were used as covariates in regression analyses with each PET scan using SPM2.
Results: Analyses revealed broad areas of the frontal lobe in which higher FCRP was associated with lower normalized glucose metabolism including the superior medial frontal, superior frontal and superior orbital frontal cortex and the ventrolateral prefrontal cortex. Significant associations were predominately found in the left hemisphere. Independent component analyses revealed interesting regions but further confirm the relevance of the integrative measure of coronary risk.
Conclusions: Although the mechanism of this association bears further investigation, this finding provides further evidence that vascular risk factors have malignant effects on the brain, particularly in the prefrontal cortex.
Figures
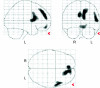
Figure Framingham Cardiovascular Risk Profile (FCRP) inversely associated with metabolism Results illustrated on SPM glass brain depicting the FCRP score negatively regressed with PET (p ≤ 0.005, uncorrected; n = 36).
Similar articles
- The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults.
DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Horwitz B, Rapoport SI, et al. DeCarli C, et al. Neurology. 1995 Nov;45(11):2077-84. doi: 10.1212/wnl.45.11.2077. Neurology. 1995. PMID: 7501162 - White matter lesions impair frontal lobe function regardless of their location.
Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, Weiner MW, Chui HC, Jagust WJ. Tullberg M, et al. Neurology. 2004 Jul 27;63(2):246-53. doi: 10.1212/01.wnl.0000130530.55104.b5. Neurology. 2004. PMID: 15277616 Free PMC article. - Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson's disease.
Mayberg HS, Starkstein SE, Sadzot B, Preziosi T, Andrezejewski PL, Dannals RF, Wagner HN Jr, Robinson RG. Mayberg HS, et al. Ann Neurol. 1990 Jul;28(1):57-64. doi: 10.1002/ana.410280111. Ann Neurol. 1990. PMID: 2375634 - Age-Related Sex-Specific Changes in Brain Metabolism and Morphology.
Kakimoto A, Ito S, Okada H, Nishizawa S, Minoshima S, Ouchi Y. Kakimoto A, et al. J Nucl Med. 2016 Feb;57(2):221-5. doi: 10.2967/jnumed.115.166439. Epub 2015 Nov 25. J Nucl Med. 2016. PMID: 26609179 - Positron emission tomography imaging of regional cerebral glucose metabolism.
Alavi A, Dann R, Chawluk J, Alavi J, Kushner M, Reivich M. Alavi A, et al. Semin Nucl Med. 1986 Jan;16(1):2-34. doi: 10.1016/s0001-2998(86)80002-2. Semin Nucl Med. 1986. PMID: 2935938 Review.
Cited by
- Higher serum total cholesterol levels in late middle age are associated with glucose hypometabolism in brain regions affected by Alzheimer's disease and normal aging.
Reiman EM, Chen K, Langbaum JB, Lee W, Reschke C, Bandy D, Alexander GE, Caselli RJ. Reiman EM, et al. Neuroimage. 2010 Jan 1;49(1):169-76. doi: 10.1016/j.neuroimage.2009.07.025. Epub 2009 Jul 23. Neuroimage. 2010. PMID: 19631758 Free PMC article. - Brain injury biomarkers are not dependent on β-amyloid in normal elderly.
Knopman DS, Jack CR Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe VJ, Kantarci K, Gunter JL, Senjem ML, Mielke MM, Roberts RO, Boeve BF, Petersen RC. Knopman DS, et al. Ann Neurol. 2013 Apr;73(4):472-80. doi: 10.1002/ana.23816. Epub 2013 Feb 19. Ann Neurol. 2013. PMID: 23424032 Free PMC article. - Irregular Baseline Brain Activity in Coronary Artery Disease Patients with Cognitive Impairment: A Resting-state Functional Magnetic Resonance Imaging Study.
Zhang J, Yan J, Niu J, Xu Z, Fang X, You J, Li T. Zhang J, et al. Curr Neurovasc Res. 2022;19(2):131-136. doi: 10.2174/1567202619666220516124552. Curr Neurovasc Res. 2022. PMID: 35578846 Free PMC article. - Vulnerable neural systems and the borderland of brain aging and neurodegeneration.
Jagust W. Jagust W. Neuron. 2013 Jan 23;77(2):219-34. doi: 10.1016/j.neuron.2013.01.002. Neuron. 2013. PMID: 23352159 Free PMC article. Review. - Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes.
Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Baker LD, et al. Arch Neurol. 2011 Jan;68(1):51-7. doi: 10.1001/archneurol.2010.225. Epub 2010 Sep 13. Arch Neurol. 2011. PMID: 20837822 Free PMC article. Clinical Trial.
References
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204. - PubMed
- Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease: The Nun Study. JAMA 1997;277:813–817. - PubMed
- Petrovitch H, Ross GW, Steinhorn SC, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol 2005;57:98–103. - PubMed
- Posner HB, Tang MX, Luchsinger J, et al. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology 2002;58:1175–1181. - PubMed
- Skoog I, Gustafson D. Update on hypertension and Alzheimer’s disease. Neurol Res 2006;28:605–611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources