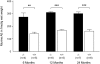Improved learning and memory in aged mice deficient in amyloid beta-degrading neutral endopeptidase - PubMed (original) (raw)
doi: 10.1371/journal.pone.0004590. Epub 2009 Feb 25.
Doris Albrecht, Matthias Becker, Manja Schubert, Elena Kouznetsova, Burkard Wiesner, Björn Maul, Reinhard Schliebs, Gisela Grecksch, Jens Furkert, Anja Sterner-Kock, Heinz-Peter Schultheiss, Axel Becker, Wolf-Eberhard Siems
Affiliations
- PMID: 19240795
- PMCID: PMC2643003
- DOI: 10.1371/journal.pone.0004590
Improved learning and memory in aged mice deficient in amyloid beta-degrading neutral endopeptidase
Thomas Walther et al. PLoS One. 2009.
Abstract
Background: Neutral endopeptidase, also known as neprilysin and abbreviated NEP, is considered to be one of the key enzymes in initial human amyloid-beta (Abeta) degradation. The aim of our study was to explore the impact of NEP deficiency on the initial development of dementia-like symptoms in mice.
Methodology/principal findings: We found that while endogenous Abeta concentrations were elevated in the brains of NEP-knockout mice at all investigated age groups, immunohistochemical analysis using monoclonal antibodies did not detect any Abeta deposits even in old NEP knockout mice. Surprisingly, tests of learning and memory revealed that the ability to learn was not reduced in old NEP-deficient mice but instead had significantly improved, and sustained learning and memory in the aged mice was congruent with improved long-term potentiation (LTP) in brain slices of the hippocampus and lateral amygdala. Our data suggests a beneficial effect of pharmacological inhibition of cerebral NEP on learning and memory in mice due to the accumulation of peptides other than Abeta degradable by NEP. By conducting degradation studies and peptide measurements in the brain of both genotypes, we identified two neuropeptide candidates, glucagon-like peptide 1 and galanin, as first potential candidates to be involved in the improved learning in aged NEP-deficient mice.
Conclusions/significance: Thus, the existence of peptides targeted by NEP that improve learning and memory in older individuals may represent a promising avenue for the treatment of neurodegenerative diseases.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Cortical Aβ deposition in brains of humans and mice.
Representative examples of immunostaining for Aβ in brain sections obtained from 6-month-old (b) and 24-month-old NEP-deficient mice (d) and corresponding age-matched wildtype controls (a,c), and from post mortem human brain material of a 75-year-old Alzheimer patient (f) and a 31-year-old human control (e). Images shown in a–d represent the mouse somatosensory cortex (barrel field), while human brain sections (e,f) were obtained from the temporal cortex. All sections were immunostained under the same conditions and in the same experimental session with the biotinylated primary antiserum 4G8 which is known to react with both human and murine Aβ peptides. Scale bar represents 200 µm.
Figure 2. Aβ accumulation in brains of NEP-deficient mice.
ELISA-measured murine Aβ(1–40) accumulation in the cortex of 6-, 12-, and 24-month old mice (−/− = NEP knockout [black bars]; +/+ = wild-type [white bars]); n≥6; average±s.e.m.; all differences were statistically calculated by a Student's t test. **P<0.01, ***P<0.001.
Figure 3. Behavioral studies in NEP-deficient mice and their wild-type controls.
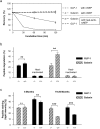
(a–c) Morris water maze: (a) Comparison of 6-month-old NEP-knockout mice (dotted line with open circles; n = 24) and their wild-type age-matched littermates (line with open quadrates; n = 19). Group means of escape latencies for the first trial per day to reach the platform ±s.e.m. in sec. Where standard variation is not visible it is within the symbols (b) Comparison of 21-month-old NEP-knockout mice (dotted line with filled circles; n = 12) and their wild-type age-matched littermates (line with filled quadrates; n = 9). Group means of escape latencies for the first trial per day to reach the platform ±s.e.m. in sec; statistical analyses by repeated measures of ANOVA (P = 0.016). (c) Comparison of time (in sec) animals needed to find the platform at both investigated time points; data were pooled for the three days of the experiment and plotted; statistical analysis by Student's t test (**P<0.01). (d) Shuttle box: group means of the number of conditioned reactions ±s.e.m. of 21-month-old NEP-knockout mice (filled bars; n = 12) and their wild-type littermates (open bars; n = 10) during a training interval on five training days in this two-way active avoidance paradigm. Statistical analysis by repeated measures of ANOVA (**P<0.01). (e) Marble burying test: Comparison of the number of marbles that have been buried by 21-month-old NEP-knockout mice (filled bars; n = 9) and their wild-type littermates (open bars; n = 11). Average±s.e.m., statistical analysis by Student's t test (*P<0.05).
Figure 4. LTP studies in NEP-deficient mice and their wild-type controls.
LTP in the hippocampus (CA1 region) and the amygdala (lateral nucleus of the amygdala (LA)) in adult (n = 7 mice) and aged NEP knockout mice (−/−; n = 7 mice) in comparison to wild-type (+/+) mice (n = 5 mice in each group). (a) 9-month-old mice: LTP magnitude (CA1: ○ +/+, n = 9 slices; • −/−, n = 11 slices; LA: ○ +/+, n = 10 slices, • −/−, n = 11 slices). Inserted representative records of PS potentials in the CA1 region before and after TBS and in the LA before and after HFS, above: obtained from −/−, below: obtained from +/+. (b) 24-month-old mice: LTP in both the CA1 region (○ +/+, n = 11 slices; • −/−, n = 16 slices) and the LA (○ +/+, n = 11 slices; • −/−, n = 10 slices). Data points in a and b represent mean amplitudes (mean±s.e.m.) normalized with respect to baseline values. Inserted representative records of PS potentials in the CA1 region before and after TBS and in the LA before and after HFS, above: obtained from −/−, below: obtained from +/+. (c) Bar histogram of data points shown in Figures 4a–b, averaged 56 to 60 min after TBS/HFS and normalized with respect to baseline (mean±s.e.m.). *P<0.05.
Figure 5. NEP-dependent neuropeptide degradation.
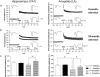
(a) HPLC-monitored degradation (recovery in %) of GLP-1 and galanin (each 5 µM) over 120 min using recombinant (rc) NEP (20 ng; R&D Systems, Wiesbaden, Germany); mean values with s.e.m.; n≥3. The reactions were stopped by adding 0.35 M perchloric acid. In parallel assays, heat-inactivated probes (5 min at 90°C) were used as a control. After centrifugation of sedimented proteins, HPLC analyses were performed by isocratic elution as described by Siems et al.. (b) HPLC-monitored peptide degradation (in %) of GLP-1 and galanin (5 µM) in brain membranes (0.5 mg protein/ml) of wild-type (+/+) and knockout mice (−/−) over 30 min. The reactions were stopped by addition of 0.35 M perchloric acid. In parallel assays, heat-inactivated NEP (5 min at 90°C) was used as a control. HPLC analysis was performed by isocratic elution as described . The significance of differences was calculated by two-sided t test; n≥4; **P<0.01. (c) For galanin and GLP-1 immunohistochemistry, slices were incubated with the specific antibodies, followed by incubation with biotinylated anti-rabbit IgG. Immunoreactive products were visualized by the nickel ammonium sulfate-intensified diaminobenzidine reaction. Unequivocally stained regions in the murine cortex were localized and defined as ROIs (regions of interest), and luminometrically compared with adjacent regions without detectable staining (reference ROIs) (for both regions n = 40). The calculated differences were shown as mean±s.e.m.). Significant differences were calculated by Student's t test, indicated by **P<0.01, ***P<0.001.
Similar articles
- Recombinant soluble neprilysin reduces amyloid-beta accumulation and improves memory impairment in Alzheimer's disease mice.
Park MH, Lee JK, Choi S, Ahn J, Jin HK, Park JS, Bae JS. Park MH, et al. Brain Res. 2013 Sep 5;1529:113-24. doi: 10.1016/j.brainres.2013.05.045. Epub 2013 Jul 2. Brain Res. 2013. PMID: 23831521 - Neprilysin deficiency alters the neuropathological and behavioral phenotype in the 5XFAD mouse model of Alzheimer's disease.
Hüttenrauch M, Baches S, Gerth J, Bayer TA, Weggen S, Wirths O. Hüttenrauch M, et al. J Alzheimers Dis. 2015;44(4):1291-302. doi: 10.3233/JAD-142463. J Alzheimers Dis. 2015. PMID: 25408216 - Neprilysin-2 is an important β-amyloid degrading enzyme.
Hafez D, Huang JY, Huynh AM, Valtierra S, Rockenstein E, Bruno AM, Lu B, DesGroseillers L, Masliah E, Marr RA. Hafez D, et al. Am J Pathol. 2011 Jan;178(1):306-12. doi: 10.1016/j.ajpath.2010.11.012. Epub 2010 Dec 23. Am J Pathol. 2011. PMID: 21224067 Free PMC article. - Aβ-degrading enzymes: potential for treatment of Alzheimer disease.
Miners JS, Barua N, Kehoe PG, Gill S, Love S. Miners JS, et al. J Neuropathol Exp Neurol. 2011 Nov;70(11):944-59. doi: 10.1097/NEN.0b013e3182345e46. J Neuropathol Exp Neurol. 2011. PMID: 22002425 Review. - Beta-amyloid catabolism: roles for neprilysin (NEP) and other metallopeptidases?
Carson JA, Turner AJ. Carson JA, et al. J Neurochem. 2002 Apr;81(1):1-8. doi: 10.1046/j.1471-4159.2002.00855.x. J Neurochem. 2002. PMID: 12067222 Review.
Cited by
- Spatially-Resolved Top-down Proteomics Bridged to MALDI MS Imaging Reveals the Molecular Physiome of Brain Regions.
Delcourt V, Franck J, Quanico J, Gimeno JP, Wisztorski M, Raffo-Romero A, Kobeissy F, Roucou X, Salzet M, Fournier I. Delcourt V, et al. Mol Cell Proteomics. 2018 Feb;17(2):357-372. doi: 10.1074/mcp.M116.065755. Epub 2017 Nov 9. Mol Cell Proteomics. 2018. PMID: 29122912 Free PMC article. - New function for an old enzyme: NEP deficient mice develop late-onset obesity.
Becker M, Siems WE, Kluge R, Gembardt F, Schultheiss HP, Schirner M, Walther T. Becker M, et al. PLoS One. 2010 Sep 16;5(9):e12793. doi: 10.1371/journal.pone.0012793. PLoS One. 2010. PMID: 20862277 Free PMC article. - The Drosophila melanogaster Neprilysin Nepl15 is involved in lipid and carbohydrate storage.
Banerjee S, Woods C, Burnett M, Park SJ, Ja WW, Curtiss J. Banerjee S, et al. Sci Rep. 2021 Jan 22;11(1):2099. doi: 10.1038/s41598-021-81165-z. Sci Rep. 2021. PMID: 33483521 Free PMC article. - A novel variant of biallelic MME gene associated with autosomal recessive late-onset distal hereditary motor neuropathy in Chinese families.
Zhang B, Gang Q, Meng L, Li Z, Chu X, Wu H, Yang J, Huang B, Du K. Zhang B, et al. BMC Med Genomics. 2024 Sep 4;17(1):223. doi: 10.1186/s12920-024-01996-3. BMC Med Genomics. 2024. PMID: 39232784 Free PMC article. - Long-term neprilysin inhibition - implications for ARNIs.
Campbell DJ. Campbell DJ. Nat Rev Cardiol. 2017 Mar;14(3):171-186. doi: 10.1038/nrcardio.2016.200. Epub 2016 Dec 15. Nat Rev Cardiol. 2017. PMID: 27974807 Review.
References
- Carson JA, Turner AJ. Beta-amyloid catabolism: roles for neprilysin (NEP) and other metallopeptidases? J Neurochem. 2002;81:1–8. - PubMed
- Tanzi RE, Moir RD, Wagner SL. Clearence of Alzheimer's Abeta peptide: the many roads of perdition. Neuron. 2004;43:605–608. - PubMed
- Turner AJ, Fisk L, Nalivaeva NN. Targeting amyloid-degrading enzymes as therapeutic strategies in neurodegeneration. Ann NY Acad Sc. 2004;1035:1–20. - PubMed
- Saido TC, Iwata N. Metabolism of amyloid beta peptide and pathogenesis of Alzheimer's disease. Towards presymptomatic diagnosis, prevention and therapy. Neurosci Res. 2006;54:235–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases