TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2 - PubMed (original) (raw)
TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2
Jeremy Copp et al. Cancer Res. 2009.
Abstract
The mammalian target of rapamycin (mTOR) serine/threonine kinase is the catalytic component of two evolutionarily conserved signaling complexes. mTOR signaling complex 1 (mTORC1) is a key regulator of growth factor and nutrient signaling. S6 kinase is the best-characterized downstream effector of mTORC1. mTOR signaling complex 2 (mTORC2) has a role in regulating the actin cytoskeleton and activating Akt through S473 phosphorylation. Herein, we show that mTOR is phosphorylated differentially when associated with mTORC1 and mTORC2 and that intact complexes are required for these mTORC-specific mTOR phosphorylations. Specifically, we find that mTORC1 contains mTOR phosphorylated predominantly on S2448, whereas mTORC2 contains mTOR phosphorylated predominantly on S2481. Using S2481 phosphorylation as a marker for mTORC2 sensitivity to rapamycin, we find that mTORC2 formation is in fact rapamycin sensitive in several cancer cell lines in which it had been previously reported that mTORC2 assembly and function were rapamycin insensitive. Thus, phospho-S2481 on mTOR serves as a biomarker for intact mTORC2 and its sensitivity to rapamycin.
Figures
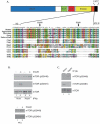
Fig. 1. mTORC1 and mTORC2 contain differentially phosphorylated mTOR
(A) Schematic of mTOR and multiple sequence alignment of the C-terminus of selected vertebrate and invertebrate TORs. Invertebrate species are Ciona intestinalis and C. savignyi (Cint and Csav); Drosophila melanogaster and D. virilis (Dmel and Dvir); Caenorhabditis elegans and C. briggsae (Cele and Cbrig); and Saccharomyces cerevisiae (TOR1 and TOR2). The region between the kinase domain (KD) and an N-terminal extension of the FATC domain (N-FATC) is conserved among vertebrates, including the marked phosphorylation sites S2448 and S2481. Asterisks indicate residues completely conserved in vertebrates. (B) HEK293 cells were serum-starved overnight. The indicated cells were stimulated with 200 nM insulin for 5 min at 37° C. Rictor and Raptor immunoprecipitates (IPs) from control and growth factor-stimulated cells were analyzed by immunoblotting with antibodies specific for mTOR phosphorylated on S2448 or S2481, or total mTOR. Whole cell lysates (WCL) were included as controls for total input. (C) Samples from actively growing U2OS cells were analyzed as in (B). Results are representative of multiple independent experiments.
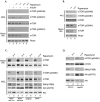
Fig. 2. Intact mTORC1 and mTORC2 are necessary for mTOR phosphorylation
(A) HEK293 cells were infected with lentiviruses expressing a control shRNA or shRNAs targeting mTOR, Raptor or Rictor. Cells were selected with puromycin 24 hr. after infection and then serum-starved overnight 2 days post-selection. The indicated cells were stimulated with 200 nM insulin for 5 min. at 37°C. WCLs were normalized for total protein concentration and were analyzed by immunoblotting with antibodies specific for mTOR phosphorylated on S2448 or S2481. Blots for total mTOR, Rictor and Raptor were included as controls. (B) HEK293 cells were infected with lentiviruses expressing a control shRNA or shRNAs targeting Rictor or mSin1. Cells were treated as in (A) and analyzed by immunoblotting for mTOR phosphorylated on S2448 or S2481. Blots for total mTOR, Rictor and mSin1 were included as controls. (C) Wild type (WT) and Sin1-/- mouse embryo fibroblasts (MEFs) were serum starved overnight. The indicated cells were stimulated with 200 nM insulin for 5 min. at 37°C. WCLs were normalized for total protein concentration and were analyzed by immunoblotting with antibodies specific for mTOR phosphorylated on S2481, total mTOR and Rictor. (D) HEK293 cells were infected with lentiviruses and were treated as in (A). Rictor and Raptor IPs from control and growth factor- stimulated cells were analyzed by immunoblotting for bound mTOR. WCLs were analyzed by immunoblotting with antibodies specific for mTOR phosphorylated on S2448 or S2481. Results are representative of multiple independent experiments.
Fig. 3. Prolonged treatment of cells with rapamycin inhibits mTOR phosphorylation on S2448 and S2481
(A) Serum-starved HEK293 cells were cultured in the presence or absence of 100 nM rapamycin for either 1 or 24 hr. The indicated cells were stimulated with 200 nM insulin for 5 min. at 37° C. WCLs were analyzed by immunoblotting with antibodies specific for mTOR phosphorylated on S2448 or S2481, or total mTOR. Rictor IPs were analyzed by immunoblotting with antibodies specific for Rictor, mTOR, and mTOR phosphorylated on S2481. (B) Actively growing U2OS cells were cultured in the presence or absence of 100 nM rapamycin for either 1 or 24 hr and analyzed as described as in (A). (C) Actively growing MDA 231, MDA 468, SKBR3 and A549 cells were treated as in (B). Rictor IPs were analyzed by immunoblotting with antibodies specific for Rictor and mTOR. WCLs were analyzed by immunoblotting for phospho-mTOR (S2481), phospho-Akt (S473), phospho-S6K (T389), total mTOR and total Akt. (D) Actively growing C2C12 myoblasts and HepG2 cells were treated as in (B) and WCLs were analyzed as in (C). Results are representative of multiple independent experiments.
Fig. 4. Depletion of mTORC2 renders S473 phosphorylation of Akt sensitive to rapamycin
MDA-MB-468 cells were infected with lentiviruses expressing a control shRNA or shRNAs targeting mTOR, Rictor or mSin1. Cells were selected with puromycin 24 hr. after infection and the indicated cells were treated with 100 nM rapamycin for an additional 24 hr. WCLs were normalized for total protein concentration and were analyzed by immunoblotting for phospho-mTOR (S2481), phospho-Akt (S473), phospho-S6K (T389), and total Akt, mTOR Rictor and mSin1. Results are representative of multiple independent experiments.
Similar articles
- mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling.
Julien LA, Carriere A, Moreau J, Roux PP. Julien LA, et al. Mol Cell Biol. 2010 Feb;30(4):908-21. doi: 10.1128/MCB.00601-09. Epub 2009 Dec 7. Mol Cell Biol. 2010. PMID: 19995915 Free PMC article. - Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2.
Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Feldman ME, et al. PLoS Biol. 2009 Feb 10;7(2):e38. doi: 10.1371/journal.pbio.1000038. PLoS Biol. 2009. PMID: 19209957 Free PMC article. - PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling.
Woo SY, Kim DH, Jun CB, Kim YM, Haar EV, Lee SI, Hegg JW, Bandhakavi S, Griffin TJ, Kim DH. Woo SY, et al. J Biol Chem. 2007 Aug 31;282(35):25604-12. doi: 10.1074/jbc.M704343200. Epub 2007 Jun 28. J Biol Chem. 2007. PMID: 17599906 - Disentangling the signaling pathways of mTOR complexes, mTORC1 and mTORC2, as a therapeutic target in glioblastoma.
Jhanwar-Uniyal M, Dominguez JF, Mohan AL, Tobias ME, Gandhi CD. Jhanwar-Uniyal M, et al. Adv Biol Regul. 2022 Jan;83:100854. doi: 10.1016/j.jbior.2021.100854. Epub 2021 Dec 6. Adv Biol Regul. 2022. PMID: 34996736 Review. - Growing knowledge of the mTOR signaling network.
Huang K, Fingar DC. Huang K, et al. Semin Cell Dev Biol. 2014 Dec;36:79-90. doi: 10.1016/j.semcdb.2014.09.011. Epub 2014 Sep 19. Semin Cell Dev Biol. 2014. PMID: 25242279 Free PMC article. Review.
Cited by
- Metformin Suppresses Development of the Echinococcus multilocularis Larval Stage by Targeting the TOR Pathway.
Loos JA, Dávila VA, Brehm K, Cumino AC. Loos JA, et al. Antimicrob Agents Chemother. 2020 Aug 20;64(9):e01808-19. doi: 10.1128/AAC.01808-19. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32540980 Free PMC article. - Experimental Approaches in Delineating mTOR Signaling.
Qian J, Su S, Liu P. Qian J, et al. Genes (Basel). 2020 Jul 2;11(7):738. doi: 10.3390/genes11070738. Genes (Basel). 2020. PMID: 32630768 Free PMC article. Review. - Distinct inhibitory effects on mTOR signaling by ethanol and INK128 in diffuse large B-cell lymphoma.
Mazan-Mamczarz K, Peroutka RJ, Steinhardt JJ, Gidoni M, Zhang Y, Lehrmann E, Landon AL, Dai B, Houng S, Muniandy PA, Efroni S, Becker KG, Gartenhaus RB. Mazan-Mamczarz K, et al. Cell Commun Signal. 2015 Mar 1;13:15. doi: 10.1186/s12964-015-0091-0. Cell Commun Signal. 2015. PMID: 25849580 Free PMC article. - Akt/mTOR integrate energy metabolism with Wnt signal to influence wound epithelium growth in Gekko Japonicus.
Wang Q, Mao Z, Liu Z, Xu M, Huang S, Wang Y, Xu Y, Qi L, Liu M, Liu Y. Wang Q, et al. Commun Biol. 2022 Sep 27;5(1):1018. doi: 10.1038/s42003-022-04004-5. Commun Biol. 2022. PMID: 36167813 Free PMC article. - Induction of apoptosis and autophagy via mitochondria- and PI3K/Akt/mTOR-mediated pathways by E. adenophorum in hepatocytes of saanen goat.
He Y, Mo Q, Luo B, Qiao Y, Xu R, Zuo Z, Deng J, Nong X, Peng G, He W, Wei Y, Hu Y. He Y, et al. Oncotarget. 2016 Aug 23;7(34):54537-54548. doi: 10.18632/oncotarget.10402. Oncotarget. 2016. PMID: 27391155 Free PMC article.
References
- Hara K, Maruki Y, Long X, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–89. - PubMed
- Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. - PubMed
- Kim DH, Sarbassov DD, Ali SM, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. - PubMed
- Haar EV, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–23. - PubMed
- Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol. 2005;16:29–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA082683/CA/NCI NIH HHS/United States
- CA82683/CA/NCI NIH HHS/United States
- P30 CA014195-35S1/CA/NCI NIH HHS/United States
- T32 CA009370/CA/NCI NIH HHS/United States
- T32 CA 09370/CA/NCI NIH HHS/United States
- R01 HG004164/HG/NHGRI NIH HHS/United States
- R01 CA082683-10/CA/NCI NIH HHS/United States
- P30 CA014195/CA/NCI NIH HHS/United States
- R01 HG004164-03/HG/NHGRI NIH HHS/United States
- CA14195/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous