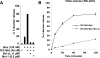Mitochondrial outer membrane proteins assist Bid in Bax-mediated lipidic pore formation - PubMed (original) (raw)
Mitochondrial outer membrane proteins assist Bid in Bax-mediated lipidic pore formation
Blanca Schafer et al. Mol Biol Cell. 2009 Apr.
Abstract
Mitochondrial outer membrane permeabilization (MOMP) is a critical step in apoptosis and is regulated by Bcl-2 family proteins. In vitro systems using cardiolipin-containing liposomes have demonstrated the key features of MOMP induced by Bax and cleaved Bid; however, the nature of the "pores" and how they are formed remain obscure. We found that mitochondrial outer membranes contained very little cardiolipin, far less than that required for liposome permeabilization, despite their responsiveness to Bcl-2 family proteins. Strikingly, the incorporation of isolated mitochondrial outer membrane (MOM) proteins into liposomes lacking cardiolipin conferred responsiveness to cleaved Bid and Bax. Cardiolipin dependence was observed only when permeabilization was induced with cleaved Bid but not with Bid or Bim BH3 peptide or oligomerized Bax. Therefore, we conclude that MOM proteins specifically assist cleaved Bid in Bax-mediated permeabilization. Cryoelectron microscopy of cardiolipin-liposomes revealed that cleaved Bid and Bax produced large round holes with diameters of 25-100 nm, suggestive of lipidic pores. In sum, we propose that activated Bax induces lipidic pore formation and that MOM proteins assist cleaved Bid in this process in the absence of cardiolipin.
Figures
Figure 1.
OMVs do not contain 7 mol % cardiolipin in their total lipids and the extracted lipids from OMVs do not support Bax-mediated membrane permeabilization. (A) Lipids were extracted from OMVs and subjected to two-dimensional TLC analysis. Cardiolipin was not visible in the expected location (circled). (B) Relative abundance of cardiolipin species in Xenopus OMV preparations detected by mass spectrometry quantification. OMVs were prepared and each cardiolipin species (based on differences in their fatty acyl chains) was quantified against the internal standard, cardiolipin C14 (see Materials and Methods). Four independently prepared OMV batches and one duplicate sample were analyzed. Numbers in parenthesis show the abundance of the respective cardiolipin species as the average mole percent of total phospholipids in the sample. The total cardiolipin content in our Xenopus OMVs was 0.26 ± 0.14 mol % (n = 5). Data shown are representative of four independent analyses. (C) OMV lipid-liposomes did not show significant levels of dextran release. Lipids were extracted from OMVs (trace amount of cardiolipin) or whole mitochondria (7 mol % cardiolipin), and liposomes were formed by the extrusion method. A dextran release assay was performed as described in Kuwana et al. (2002). Data shown are representative of two independent experiments. (D) A list of vesicles used in this study.
Figure 2.
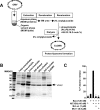
OMVs possess components that facilitate N/C-Bid and Bax-induced membrane permeabilization. (A) Schematic representation of the protocol for generating liposomes by detergent-mediated method (left). Dried lipids were resuspended in buffer containing 2–4% OG, and liposomes (OG-LUVs) were formed as detergent was removed by dialysis. ReOMVs were formed based on the same principle (right) from detergent-solubilized OMVs. ReOMVs were made solely from endogenous OMV lipids and proteins. (B) ReOMVs responded to Bcl-2 family proteins in the same way that cardiolipin-containing liposomes do; Bax and N/C-Bid released dextrans and the release was inhibited by antiapoptotic Bcl-xL or Mcl-1. Note that N/C-Bid alone did not permeabilize the vesicles. (C) ReLMVs, generated from detergent-solubilized LMVs, were not permeabilized significantly in the presence of N/C-Bid and Bax. (D) The bacterial outer membrane protein OmpA did not confer responsiveness to liposomes or ReLMVs. Data shown are representative of four independent experiments. (E) OmpA loading did not interfere with the responsiveness of ReOMVs. (F) Coomassie Blue-stained SDS-PAGE gel confirms the loading of membrane proteins in ReLMVs, ReOMVs + OmpA, ReOMVs, and ReLMVs + OmpA. Prominent proteins such as VDAC (a doublet in Xenopus) and OmpA are indicated with arrows.
Figure 3.
MOM proteins facilitate Bax-mediated membrane permeabilization. (A) Schematic diagram for the generation of ExOMVs. OMVs were mixed with organic solvents to extract the lipids. The membrane proteins, which had partitioned into the aqueous phase, were denatured in 8 M urea, and then allowed to refold in detergent by dilution and dialysis. A mixture of purified phospholipids (Avanti Polar Lipids) was mixed with the refolded proteins in detergent. OG was then removed by dialysis to allow the formation of proteo-liposomes composed of MOM proteins and exogenous phospholipids (ExOMVs). The lipid composition of PC:PE:PI:PS, 59:23:16:2 mol %, mimics that of OMVs. (B) MOM proteins were loaded in ExOMVs. An aliquot of the sample from each step was loaded and stained by Coomassie Blue. (C) ExOMVs were permeabilized by N/C-Bid and Bax, and this release was inhibited by Bcl-xL or Mcl-1. ExOMVs exhibited the same characteristics as cardiolipin-containing liposomes and ReOMVs. Data shown are representative of five experiments.
Figure 4.
Cardiolipin dependence is mainly conferred by N/C-Bid. (A) Liposomes containing 0 or 7% mol cardiolipin were generated and dextran release assays were performed with different activators of Bax, namely, N/C-Bid, Bid BH3 peptide, or Bim BH3 peptide. Among those, only N/C-Bid showed cardiolipin dependence. The peptides alone did not permeabilize liposomes (data not shown). Data shown are representative of four experiments. (B) OG-oligomerized Bax (OG-Bax) permeabilized liposomes in the absence of cardiolipin to a significant degree. Data shown are representative of two experiments. (C) Mtch2, a reported Bid-binding protein in the MOM (Grinberg et al., 2005), was detected in ReOMVs but not in ExOMVs. ReOMVs and ExOMVs were loaded on to a SDS-PAGE gel, and proteins were stained by Coomassie Blue. Predominant bands ∼30-kDa were subjected to trypsin digestion, and the proteins were identified by mass spectrometry. Note that Xenopus VDAC (xVDAC) variants xVDAC1 and 2 do not correspond to the mammalian VDAC isoforms.
Figure 5.
OG-LUVs respond to recombinant Bcl-2 family proteins. (A) Dextrans were released from OG-LUVs containing 7 mol % cardiolipin. N/C-Bid and Bax permeabilized the vesicles, and the release was inhibited by the addition of the anti-apoptotic protein Bcl-xL or Mcl-1. N/C-Bid and Bax-mediated release from OG-LUVs was cardiolipin dependent (data not shown). (B) Release from OG-LUVs was monitored at 10, 30, 60, and 120 min. In the presence of Bax (120 nM) and N/C-Bid (45 nM), the dextran release from a population of vesicles reached a plateau at 1 h. This release was inhibited when Bcl-xL (1 μM) was also present. Data shown are representative of three independent experiments.
Figure 6.
N/C-Bid and Bax induce pore-like structures in the OG-LUV membrane. (A) Nontreated OG-LUVs seemed perfectly round and had smooth and continuous membranes. Vesicles were instantaneously frozen in vitrified ice and visualized by cryo-EM. (B) After a 5-min incubation with N/C-Bid and Bax, the liposome membranes exhibited regions of irregularity and some pore-like structures were visible (arrows). (C) After 60 min of incubation with N/C-Bid and Bax, almost all the vesicles exhibited membrane abnormalities (arrows). (D) In the presence of Bcl-xL, the presence of the pores and abnormal membranes were greatly diminished.
Figure 7.
(A) A gallery of pore-bearing vesicles and a cartoon illustration of how the pores may be formed (bottom). Bar, 100 nm. (B) Views of two permeabilized vesicles (liposome 1 and 2) with tilted angles: −45°, 0°, and +45°. (C) Summary of the number of porous vesicles over time. The percentages of vesicles with pores correlated well with dextran-release shown in Figure 5B. the total number of counted vesicles is shown in parentheses.
Similar articles
- Distinct lipid effects on tBid and Bim activation of membrane permeabilization by pro-apoptotic Bax.
Shamas-Din A, Bindner S, Chi X, Leber B, Andrews DW, Fradin C. Shamas-Din A, et al. Biochem J. 2015 May 1;467(3):495-505. doi: 10.1042/BJ20141291. Biochem J. 2015. PMID: 25714678 - The cytosolic domain of Bcl-2 forms small pores in model mitochondrial outer membrane after acidic pH-induced membrane association.
Peng J, Lapolla SM, Zhang Z, Lin J. Peng J, et al. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009 Feb;26(1):130-7. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009. PMID: 19334571 Free PMC article. - Involvement of cardiolipin in tBID-induced activation of BAX during apoptosis.
Raemy E, Martinou JC. Raemy E, et al. Chem Phys Lipids. 2014 Apr;179:70-4. doi: 10.1016/j.chemphyslip.2013.12.002. Epub 2013 Dec 12. Chem Phys Lipids. 2014. PMID: 24333953 Review. - Bid: a Bax-like BH3 protein.
Billen LP, Shamas-Din A, Andrews DW. Billen LP, et al. Oncogene. 2008 Dec;27 Suppl 1:S93-104. doi: 10.1038/onc.2009.47. Oncogene. 2008. PMID: 19641510 Review.
Cited by
- Pro-apoptotic Bax molecules densely populate the edges of membrane pores.
Kuwana T, Olson NH, Kiosses WB, Peters B, Newmeyer DD. Kuwana T, et al. Sci Rep. 2016 Jun 3;6:27299. doi: 10.1038/srep27299. Sci Rep. 2016. PMID: 27255832 Free PMC article. - Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics.
Martinou JC, Youle RJ. Martinou JC, et al. Dev Cell. 2011 Jul 19;21(1):92-101. doi: 10.1016/j.devcel.2011.06.017. Dev Cell. 2011. PMID: 21763611 Free PMC article. Review. - Anti-apoptotic activity and proteasome-mediated degradation of Xenopus Mcl-1 protein in egg extracts.
Tsuchiya Y, Yamashita S. Tsuchiya Y, et al. J Biol Chem. 2011 May 6;286(18):15806-14. doi: 10.1074/jbc.M110.175927. Epub 2011 Mar 17. J Biol Chem. 2011. PMID: 21454490 Free PMC article. - Reconstitution of proapoptotic BAK function in liposomes reveals a dual role for mitochondrial lipids in the BAK-driven membrane permeabilization process.
Landeta O, Landajuela A, Gil D, Taneva S, DiPrimo C, Sot B, Valle M, Frolov VA, Basañez G. Landeta O, et al. J Biol Chem. 2011 Mar 11;286(10):8213-8230. doi: 10.1074/jbc.M110.165852. Epub 2011 Jan 1. J Biol Chem. 2011. PMID: 21196599 Free PMC article. - MOMP, cell suicide as a BCL-2 family business.
Kalkavan H, Green DR. Kalkavan H, et al. Cell Death Differ. 2018 Jan;25(1):46-55. doi: 10.1038/cdd.2017.179. Epub 2017 Oct 20. Cell Death Differ. 2018. PMID: 29053143 Free PMC article. Review.
References
- Almgren M. Mixed micelles and other structures in the solubilization of bilayer lipid membranes by surfactants. Biochim. Biophys. Acta. 2000;1508:146–163. - PubMed
- Antonsson B., Montessuit S., Sanchez B., Martinou J. C. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 2001;276:11615–11623. - PubMed
- Ardail D., Privat J. P., Egret-Charlier M., Levrat C., Lerme F., Louisot P. Mitochondrial contact sites. Lipid composition and dynamics. J. Biol. Chem. 1990;265:18797–18802. - PubMed
- Arnold T., Poynor M., Nussberger S., Lupas A. N., Linke D. Gene duplication of the eight-stranded beta-barrel OmpX produces a functional pore: a scenario for the evolution of transmembrane beta-barrels. J. Mol. Biol. 2007;366:1174–1184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21AG024157/AG/NIA NIH HHS/United States
- R21 AG024157/AG/NIA NIH HHS/United States
- P41 RR017573/RR/NCRR NIH HHS/United States
- F32 CA101444/CA/NCI NIH HHS/United States
- R21 AG024478/AG/NIA NIH HHS/United States
- RR17573/RR/NCRR NIH HHS/United States
- R21AG024478/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials