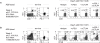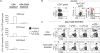Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade - PubMed (original) (raw)
Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade
Nobuhiro Nakamoto et al. PLoS Pathog. 2009 Feb.
Abstract
Viral persistence is associated with hierarchical antiviral CD8 T cell exhaustion with increased programmed death-1 (PD-1) expression. In HCV persistence, HCV-specific CD8 T cells from the liver (the site of viral replication) display increased PD-1 expression and a profound functional impairment that is not reversed by PD-1 blockade alone. Here, we report that the inhibitory receptor cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) is preferentially upregulated in PD-1(+) T cells from the liver but not blood of chronically HCV-infected patients. PD-1/CTLA-4 co-expression in intrahepatic T cells was associated with a profound HCV-specific effector dysfunction that was synergistically reversed by combined PD-1/CTLA-4 blockade in vitro, but not by blocking PD-1 or CTLA-4 alone. A similar effect was observed in circulating HCV-specific CD8 T cells with increased PD-1/CTLA-4 co-expression during acute hepatitis C. The functional response to combined blockade was directly associated with CTLA-4 expression, lost with CD28-depletion and CD4-independent (including CD4(+)FoxP3(+) Tregs). We conclude that PD-1 and CTLA-4 pathways both contribute to virus-specific T cell exhaustion at the site of viral replication by a redundant mechanism that requires combined PD-1/CTLA-4 blockade to reverse. These findings provide new insights into the mechanisms of virus-specific T cell dysfunction, and suggest that the synergistic effect by combined inhibitory receptor blockade might have a therapeutic application against chronic viral infection in vivo, provided that it does not induce autoimmunity.
Conflict of interest statement
G.J.F. has a patent licensing arrangement for antibodies blocking the PD-1/PD-L pathways.
Figures
Figure 1. CTLA-4 expression is increased in intrahepatic HCV-specific CD8 T cells.
(A) %CTLA-4+ in CD8 T cells from 29 chronic (C), 6 acute (A) and 4 resolved (R) hepatitis C patients and 10 HCV-seronegative controls (N). Median: C (blood 1.6%, liver 6.4%); R 1.3%; N 0.9%; A 10.6%. (B) CTLA-4 expression in tetramer+ CD8 T cells specific for HLA-A2-restricted HCV (NS3 1073, NS3 1406, NS5 2594) and non-HCV (Flu, CMV and EBV) epitopes from 11C, 3R and 4A patients. Median CTLA-4 MFI: C (blood: HCV 77, non-HCV 61; liver: HCV 151, non-HCV 43). Median %CTLA-4+: C (blood: HCV 1.4%, non-HCV 0.9%; liver: HCV 22.2%, non-HCV 0.6%); R (HCV 0.6%, non-HCV 0%); A (HCV 14.0%, non-HCV 2.9%). Red horizontal bars indicate median value. P-values were determined by Mann-Whitney U test. (C) Representative flow cytometry plots. (Top): Staining characteristics of tetramer+ CD8 T cells. (Middle): PD-1/CTLA-4 staining of gated tetramer+ CD8 T cells (dot plots). (Bottom): PD-1 and CTLA-4 cutoff strategy based on the isotype. (D) Representative FACS plots showing preferential CTLA-4 expression in PD-1-high cells (left) and cutoff strategy based on the isotype (left) in intrahepatic CD8-gated T cells demonstrated with PE-conjugated αPD-1 mAb. (E) Correlation between PD-1 and CTLA-4 expressions in HCV-specific tetramer+ CD8 T cells ex vivo from HCV-seropositive subjects. Red circles: HCV-specific CD8 T cells from HCV-infected liver and peripheral blood of acute HCV patients.
Figure 2. PD-1+CTLA-4+ CD8 T cells from HCV-infected liver highly express CD28 but not ICOS or BTLA.
(A) CD28 expression by percentage and MFI in total CD8 T cells from blood and in liver-derived CD8 T cell subsets (PD-1−CTLA-4−, PD-1+CTLA-4−, PD-1+CTLA-4+) ex vivo from 18 chronic HCV patients. Median values (red horizontal lines): %CD28+ in intrahepatic PD-1−CTLA-4− vs. PD-1+CTLA-4− vs. PD-1+CTLA-4+ subsets (33% vs. 49% vs. 62%, p<0.0001 by the Kruskal Wallis test). (B) BTLA and ICOS expressions by percentage and MFI in total CD8 T cells from blood and in liver-derived CD8 T cell subsets (PD-1−CTLA-4−, PD-1+CTLA-4−, PD-1+CTLA-4+) ex vivo from 5 chronic HCV patients. Median values (red horizontal lines): %BTLA+ (intrahepatic CD8 subsets: 0.1% vs. 0.4% vs. 0.3%, p = 0.364); %ICOS+ (intrahepatic CD8 subsets: 0.1% vs. 0.5% vs. 2.4%, p = 0.049); ICOS MFI (intrahepatic CD8 subsets: 43 vs. 50 vs. 78, p = 0.021). The p-values were calculated by the Kruskal Wallis test. Flow cytometry plots on the right show the characteristic PD-1 and CTLA-4 expression in intrahepatic CD8 T cells (left) and the relative CD28, BTLA and ICOS expression in PD-1+CTLA-4+ (Red), PD-1+CTLA-4− (Green) and PD-1−CTLA-4− (Blue) CD8 T cell subsets relative to isotype control (gray shade) in the histograms on the right.
Figure 3. Intrahepatic HCV-specific T cell dysfunction can be reversed synergistically by combined PD-1/CTLA-4 blockade.
(A) Effect of inhibitory receptor blockade on HCV-specific IFN-γ and TNF-α production by CD8 and CD4 T cells from liver and blood. The bar graphs show the frequency of CD8 (upper graphs) and CD4 (lower graphs) T cells with HCV-specific intracellular IFN-γ and/or TNF-α expression in liver-derived (left panel, n = 6) and blood-derived (right panel, n = 8) lymphocytes isolated from chronic HCV patients and cultured for 7 days in vitro with 15mer overlapping peptides spanning the entire HCV NS3 protein (pNS3) in the presence of isotype control or blocking antibodies. The 7-day cultures were further stimulated for 6 hours with media alone or with pNS3 before intracellular IFN-γ and TNF-α staining. The %IFN-γ−TNF-α+ (blue bar), %IFN-γ+TNF-α+ (red bar) and %IFN-γ+TNF-α− (yellow bar) T cells are stacked together in each case to show total cytokine+ cells. (B) Representative flow cytometry plots showing HCV NS3 and Flu-specific IFN-γ and TNF-α production in vitro in liver-derived CD8 T cells from chronic HCV patient C21 following 7 days of culture with NS3 or Flu-derived peptides in the presence of isotype or blocking antibodies. Flow cytometry plots on the far left shows the PD-1 and CTLA-4 expression in liver-derived CD8 T cells directly ex vivo.
Figure 4. Effect of PD-1/CTLA-4 blockade on intrahepatic and peripheral HCV-specific tetramer+ CD8 T cell function.
Flow cytometry plots showing HCV 1073-specific CD8 T cell phenotype directly ex vivo and antigen-specific functions following 7 days of antigenic stimulation in the presence of isotype or blocking antibodies, using liver-derived (A) and blood-derived (B) lymphocytes from chronic patient C57. (Top panels): frequency of HCV 1073-specific CD8 T cells determined by cognate HLA-A2 tetramer staining. (Middle panels) far left: PD-1 and CTLA-4 expression ex vivo in gated tetramer+ CD8 T cells (dot plots). Remaining right panels: HCV-specific IFN-γ production and CD107a mobilization in gated tetramer+ CD8 T cells on day 7. (Bottom panels): Perforin expression in tetramer+ (blue line) and total CD8 T cells (gray shaded) on day 7. (C) Fold increase in the expansion and effector functions of liver-derived (left) and blood-derived (right) HCV-specific CD8 T cells by αPD-L1 alone (white bar), αCTLA-4 alone (gray bar) and combined αPD-L1/αCTLA-4 blockade (black bar) relative to the isotype control for 3 chronic patients. The frequencies of functional tetramer+ CD8 T cells in each culture were calculated by multiplying %tetramer+ CD8 T cells with %IFN-γ+/tetramer+ CD8 T cells, %perforin+/tetramer+ CD8 T cells or %CD107a+/tetramer+ CD8 T cells. (D) Flow cytometry plots showing CMV-specific CD8 T cells directly ex vivo and their antigen-specific functions following 7 days in vitro cultures from chronic patient C99.
Figure 5. PD-1/CTLA-4 blockade can rejuvenate circulating PD-1+CTLA-4+ HCV-specific CD8 T cells during acute hepatitis C.
(Left panels): Flow cytometry plots showing peripheral HCV-specific tetramer+ CD8 T cells during acute hepatitis C in patients A29 (A) and A35 (B) ex vivo. Gated tetramer+ CD8 T cells (dot plots) exhibit increased PD-1 and CTLA-4 expression. (Right panels): Flow cytometry plots showing HCV-specific tetramer+ CD8 T cell frequency following 7 days of culture with antigenic peptide in the presence of isotype or blocking antibodies for A29. In A35, HCV-specific tetramer+ CD8 T cell proliferation was directly monitored in CFSE dilution assay (gating on CD8 T cells) in which the events on the left upper quadrant represent tetramer+ CD8 T cells that expanded with CFSE-dilution.
Figure 6. The functional response to PD-1/CTLA-4 blockade associate directly with CTLA-4 expression but not FoxP3+ Tregs.
(A) Correlation between HCV specific effector cytokine response to combined PD-1/CTLA-4 blockade and ex vivo %CTLA-4+/CD8 but not %PD-1+/CD8 and %FoxP3+/CD4. The y-axis represents the sum of CD8 T cells with HCV-specific IFN-γ+TNF-α+, IFN-γ+TNF-α− and IFN-γ−TNF-α+ expression during combined PD-1/CTLA-4 blockade from 14 HLA-A2− patients (6 intrahepatic and 8 peripheral blood responses). R and p-values by the Spearman rank correlation test. (B) Positive correlation between fold expansion of HCV-specific tetramer+ CD8 T cells with combined PD-1/CTLA-4 blockade (relative to PD-1 blockade alone) and ex vivo %CTLA-4+ in HCV-specific tetramer+ CD8 T cells in 7 HLA A2+ HCV-infected patients. R- and p-values by the Spearman rank correlation test. (C) (Left): Liver infiltrating lymphocytes from chronic patient C07 are examined for CD4, CD8 and FoxP3+ T cell subsets before (upper) and after (lower) depletion of CD4 T cells by CD4 Dynabeads (Dynal Inc), resulting in >99% depletion of CD4 T cells including FoxP3+ CD4 T cells. (Right): Undepleted and CD4-depleted liver infiltrating lymphocytes were cultured for 7 days with overlapping HCV NS3-derived 15mer peptides (pNS3) in the presence of isotype or blocking antibodies before direct staining for T cell subsets (CD4, FoxP3) and following additional 6 hours of stimulation with media alone (negative control) or pNS3 peptides to examine HCV-specific intracellular IFN-γ and TNF-α expression in CD8 T cells. Combined PD-1/CTLA-4 blockade promoted markedly enhanced HCV-specific cytokine response in undepleted and CD4-depleted cultures regardless of FoxP3+ Tregs.
Figure 7. The functional restoration by PD-1/CTLA-4 blockade is CD28-dependent.
(A) Loss of HCV-specific CD8 T-cell IFN-γ response by CD28 depletion. LIL or PBL from 3 HLA-A2-negative patients with chronic (C08, C275) and acute (A36) hepatitis C were depleted of CD4 without or with additional CD28 depletion before in vitro culture for 7 days with HCV NS3-derived overlapping 15mer peptides in the presence of isotype or PD-1/CTLA-4 blockade. Cultured cells were examined for HCV-specific IFN-γ production in a 45 hour IFN-γ ELISPOT assay. (B) CD28 expression in HCV-specific NS3 1073-specific tetramer+ CD8 T-cells relative to PD-1 and CTLA-4 expression ex vivo. (left) Peripheral HCV 1073-specific tetramer+ CD8 T cells from an HLA-A2+ acute HCV patient (A47) display CD28 expression in 28%. (middle) Gated HCV 1073-specific tetramer+ CD8 T cells show the characteristic PD-1 (97.3%) and CTLA-4 (20.5%) expression. (right) Increased CD28 expression in gated PD-1+CTLA-4+ (Red; 50.0%) HCV tetramer+ CD8 T cells compared to PD-1+CTLA-4− (Green; 19.5%) and PD-1−CTLA-4− (Blue; 12.2%) subsets and isotype control (gray shade) in histogram. (C) Effect of CD28-depletion on antigen-specific expansion in the presence of PD-1 and/or CTLA-4 blockade is shown by CFSE-dilution for HCV NS3 1073-specific tetramer+ CD8 T cells from patient A47. CD4 depleted PBL with and without CD28-depletion were CFSE-labeled and stimulated for 7 days in vitro with HCV NS3 1073 peptide in the presence of isotype or blocking antibodies before flow cytometric analysis. Note that HCV tetramer+ CD8 T cells remain detectable with CD28 depletion in the bottom graphs.
Similar articles
- Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization.
Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Nakamoto N, et al. Gastroenterology. 2008 Jun;134(7):1927-37, 1937.e1-2. doi: 10.1053/j.gastro.2008.02.033. Epub 2008 Feb 17. Gastroenterology. 2008. PMID: 18549878 Free PMC article. - Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction.
Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Golden-Mason L, et al. J Virol. 2007 Sep;81(17):9249-58. doi: 10.1128/JVI.00409-07. Epub 2007 Jun 13. J Virol. 2007. PMID: 17567698 Free PMC article. - Liver environment and HCV replication affect human T-cell phenotype and expression of inhibitory receptors.
Kroy DC, Ciuffreda D, Cooperrider JH, Tomlinson M, Hauck GD, Aneja J, Berger C, Wolski D, Carrington M, Wherry EJ, Chung RT, Tanabe KK, Elias N, Freeman GJ, de Kruyff RH, Misdraji J, Kim AY, Lauer GM. Kroy DC, et al. Gastroenterology. 2014 Feb;146(2):550-61. doi: 10.1053/j.gastro.2013.10.022. Epub 2013 Oct 19. Gastroenterology. 2014. PMID: 24148617 Free PMC article. - Costimulatory molecule programmed death-1 in the cytotoxic response during chronic hepatitis C.
Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Larrubia JR, et al. World J Gastroenterol. 2009 Nov 7;15(41):5129-40. doi: 10.3748/wjg.15.5129. World J Gastroenterol. 2009. PMID: 19891011 Free PMC article. Review. - Programmed Cell Death 1 (PD-1) and Cytotoxic T Lymphocyte-Associated Antigen 4 (CTLA-4) in Viral Hepatitis.
Cho H, Kang H, Lee HH, Kim CW. Cho H, et al. Int J Mol Sci. 2017 Jul 13;18(7):1517. doi: 10.3390/ijms18071517. Int J Mol Sci. 2017. PMID: 28703774 Free PMC article. Review.
Cited by
- Clearance of Hepatitis C Virus following Immune Checkpoint Inhibitor Therapy for Hepatocellular Carcinoma: Case Report.
Wilson H, Macdonald D, Bryce K. Wilson H, et al. Case Rep Gastroenterol. 2024 Jun 27;18(1):347-351. doi: 10.1159/000539646. eCollection 2024 Jan-Dec. Case Rep Gastroenterol. 2024. PMID: 39015527 Free PMC article. - Single-cell RNA-seq reveals T cell exhaustion and immune response landscape in osteosarcoma.
Fan Q, Wang Y, Cheng J, Pan B, Zang X, Liu R, Deng Y. Fan Q, et al. Front Immunol. 2024 Apr 2;15:1362970. doi: 10.3389/fimmu.2024.1362970. eCollection 2024. Front Immunol. 2024. PMID: 38629071 Free PMC article. - The expression of CTLA-4 in hepatic alveolar echinococcosis patients and blocking CTLA-4 to reverse T cell exhaustion in _Echinococcus multilocularis_-infected mice.
Yang Y, Wuren T, Wu B, Cheng S, Fan H. Yang Y, et al. Front Immunol. 2024 Mar 28;15:1358361. doi: 10.3389/fimmu.2024.1358361. eCollection 2024. Front Immunol. 2024. PMID: 38605966 Free PMC article. - In vivo single-cell high-dimensional mass cytometry analysis to track the interactions between Klebsiella pneumoniae and myeloid cells.
Calderon-Gonzalez R, Dumigan A, Sá-Pessoa J, Kissenpfennig A, Bengoechea JA. Calderon-Gonzalez R, et al. PLoS Pathog. 2024 Apr 5;20(4):e1011900. doi: 10.1371/journal.ppat.1011900. eCollection 2024 Apr. PLoS Pathog. 2024. PMID: 38578798 Free PMC article. - Possibility of PD-1/PD-L1 Inhibitors for the Treatment of Patients with Chronic Hepatitis B Infection.
Su M, Ye T, Wu W, Shu Z, Xia Q. Su M, et al. Dig Dis. 2024;42(1):53-60. doi: 10.1159/000534535. Epub 2023 Oct 11. Dig Dis. 2024. PMID: 37820605 Free PMC article. Review.
References
- Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol. 2007;178:2714–2720. - PubMed
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI047519/AI/NIAID NIH HHS/United States
- P30 DK050306-11/DK/NIDDK NIH HHS/United States
- M01 RR00040/RR/NCRR NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- P30 DK50306/DK/NIDDK NIH HHS/United States
- P30 DK050306-13/DK/NIDDK NIH HHS/United States
- P30 CA016520-229012/CA/NCI NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- AI47519/AI/NIAID NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- M01 RR000040/RR/NCRR NIH HHS/United States
- P30 DK050306-12/DK/NIDDK NIH HHS/United States
- P01 AI056299-019001/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials