Insulin granule biogenesis, trafficking and exocytosis - PubMed (original) (raw)
Review
Insulin granule biogenesis, trafficking and exocytosis
June Chunqiu Hou et al. Vitam Horm. 2009.
Abstract
It is becoming increasingly apparent that beta cell dysfunction resulting in abnormal insulin secretion is the essential element in the progression of patients from a state of impaired glucose tolerance to frank type 2 diabetes (Del Prato, 2003; Del Prato and Tiengo, 2001). Although extensive studies have examined the molecular, cellular and physiologic mechanisms of insulin granule biogenesis, sorting, and exocytosis the precise mechanisms controlling these processes and their dysregulation in the developed of diabetes remains an area of important investigation. We now know that insulin biogenesis initiates with the synthesis of preproinsulin in rough endoplastic reticulum and conversion of preproinsulin to proinsulin. Proinsulin begins to be packaged in the Trans-Golgi Network and is sorting into immature secretory granules. These immature granules become acidic via ATP-dependent proton pump and proinsulin undergoes proteolytic cleavage resulting the formation of insulin and C-peptide. During the granule maturation process, insulin is crystallized with zinc and calcium in the form of dense-core granules and unwanted cargo and membrane proteins undergo selective retrograde trafficking to either the constitutive trafficking pathway for secretion or to degradative pathways. The newly formed mature dense-core insulin granules populate two different intracellular pools, the readily releasable pools (RRP) and the reserved pool. These two distinct populations are thought to be responsible for the biphasic nature of insulin release in which the RRP granules are associated with the plasma membrane and undergo an acute calcium-dependent release accounting for first phase insulin secretion. In contrast, second phase insulin secretion requires the trafficking of the reserved granule pool to the plasma membrane. The initial trigger for insulin granule fusion with the plasma membrane is a rise in intracellular calcium and in the case of glucose stimulation results from increased production of ATP, closure of the ATP-sensitive potassium channel and cellular depolarization. In turn, this opens voltage-dependent calcium channels allowing increased influx of extracellular calcium. Calcium is thought to bind to members of the fusion regulatory proteins synaptogamin that functionally repressors the fusion inhibitory protein complexin. Both complexin and synaptogamin interact as well as several other regulatory proteins interact with the core fusion machinery composed of the Q- or t-SNARE proteins syntaxin 1 and SNAP25 in the plasma membrane that assembles with the R- or v-SNARE protein VAMP2 in insulin granules. In this chapter we will review the current progress of insulin granule biogenesis, sorting, trafficking, exocytosis and signaling pathways that comprise the molecular basis of glucose-dependent insulin secretion.
Figures
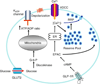
Figure 16.1
Insulin biogenesis: Preproinsulin is synthesized in rough endoplasmic reticulum (RER) and is converted to proinsulin. Proinsulin is later transported to Trans-Golgi Network (TGN) and packed into immature granule via sorting for entry. The immature granule loses the clathrin coating and other unwanted components through sorting by exit. In parallel, proinsulin is converted to insulin and condensation/crystallization (sorting by retention) occurs. These processes account for the maturation of the immature insulin granules into a mature insulin secretory granule following TGN exit.
Figure 16.2
Signaling pathway: Glucose, the major stimulant, via glycolysis and mitochondrial ATP energy production increases the ATP/ADP ratio that leads to the closure of the ATP-sensitive K+ (KATP) channels. The subsequent cellular depolarization activates voltage dependent calcium channels resulting in extracellular Ca2+ influx and fusion of insulin granules with the plasma membrane. The incretin hormone GLP-1 acts on its receptor at β cell plasma membrane to activate adenylyl cyclase and increase intracellular cAMP levels. In turn, cAMP binds and activates protein kinase A and EPAC. EPAC then functions to increase intracellular calcium level from intracellular calcium stores in the endoplasmic reticulum and to increase the number of readily releasable pool of insulin granules at the plasma membrane. The combination of these two processes results in a potentiation of insulin secretion.
Similar articles
- A review on insulin trafficking and exocytosis.
Vakilian M, Tahamtani Y, Ghaedi K. Vakilian M, et al. Gene. 2019 Jul 20;706:52-61. doi: 10.1016/j.gene.2019.04.063. Epub 2019 Apr 27. Gene. 2019. PMID: 31039435 Review. - Glucose-stimulated signaling pathways in biphasic insulin secretion.
Straub SG, Sharp GW. Straub SG, et al. Diabetes Metab Res Rev. 2002 Nov-Dec;18(6):451-63. doi: 10.1002/dmrr.329. Diabetes Metab Res Rev. 2002. PMID: 12469359 Review. - TRP Channel Trafficking.
Planells-Cases R, Ferrer-Montiel A. Planells-Cases R, et al. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. PMID: 21204515 Free Books & Documents. Review. - Regulation of secretory granule recruitment and exocytosis at rat neurohypophysial nerve endings.
Giovannucci DR, Stuenkel EL. Giovannucci DR, et al. J Physiol. 1997 Feb 1;498 ( Pt 3)(Pt 3):735-51. doi: 10.1113/jphysiol.1997.sp021898. J Physiol. 1997. PMID: 9051585 Free PMC article. - Rab2a and Rab27a cooperatively regulate the transition from granule maturation to exocytosis through the dual effector Noc2.
Matsunaga K, Taoka M, Isobe T, Izumi T. Matsunaga K, et al. J Cell Sci. 2017 Feb 1;130(3):541-550. doi: 10.1242/jcs.195479. Epub 2016 Dec 7. J Cell Sci. 2017. PMID: 27927751
Cited by
- New roles of carboxypeptidase E in endocrine and neural function and cancer.
Cawley NX, Wetsel WC, Murthy SR, Park JJ, Pacak K, Loh YP. Cawley NX, et al. Endocr Rev. 2012 Apr;33(2):216-53. doi: 10.1210/er.2011-1039. Epub 2012 Mar 7. Endocr Rev. 2012. PMID: 22402194 Free PMC article. Review. - PI3K p110α/Akt signaling negatively regulates secretion of the intestinal peptide neurotensin through interference of granule transport.
Li J, Song J, Cassidy MG, Rychahou P, Starr ME, Liu J, Li X, Epperly G, Weiss HL, Townsend CM Jr, Gao T, Evers BM. Li J, et al. Mol Endocrinol. 2012 Aug;26(8):1380-93. doi: 10.1210/me.2012-1024. Epub 2012 Jun 14. Mol Endocrinol. 2012. PMID: 22700584 Free PMC article. - Non-toxic fragment of botulinum neurotoxin type A and monomethyl auristatin E conjugate for targeted therapy for neuroendocrine tumors.
Whitt J, Hong WS, Telange RR, Lin CP, Bibb J, Beebe DJ, Chen H, Jaskula-Sztul R. Whitt J, et al. Cancer Gene Ther. 2020 Dec;27(12):898-909. doi: 10.1038/s41417-020-0167-x. Epub 2020 Feb 7. Cancer Gene Ther. 2020. PMID: 32029905 - Identification of Vesicle-Mediated Transport-Related Genes for Predicting Prognosis, Immunotherapy Response, and Drug Screening in Cervical Cancer.
Lou S, Lv H, Zhang L. Lou S, et al. Immun Inflamm Dis. 2024 Nov;12(11):e70052. doi: 10.1002/iid3.70052. Immun Inflamm Dis. 2024. PMID: 39513664 Free PMC article. - Deconstructing pancreas developmental biology.
Benitez CM, Goodyer WR, Kim SK. Benitez CM, et al. Cold Spring Harb Perspect Biol. 2012 Jun 1;4(6):a012401. doi: 10.1101/cshperspect.a012401. Cold Spring Harb Perspect Biol. 2012. PMID: 22587935 Free PMC article. Review.
References
- Abderrahmani A, Niederhauser G, Plaisance V, Roehrich ME, Lenain V, Coppola T, Regazzi R, Waeber G. Complexin I regulates glucose-induced secretion in pancreatic beta-cells. J. Cell Sci. 2004;117:2239–2247. - PubMed
- Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphatesensitive potassium channels. Endocr. Rev. 1999;20:101–135. - PubMed
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JPt, Boyd AE, 3rd, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: A regulator of insulin secretion. Science. 1995;268:423–426. - PubMed
- Ammon HP, Steinke J. 6-Amnionicotinamide (6-AN) as a diabetogenic agent. In vitro and in vivo studies in the rat. Diabetes. 1972a;21:143–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical