Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine - PubMed (original) (raw)
Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine
Silvia Fre et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Notch and Wnt signals play essential roles in intestinal development and homeostasis, yet how they integrate their action to affect intestinal morphogenesis is not understood. We examined the interplay between these two signaling pathways in vivo, by modulating Notch activity in mice carrying either a loss- or a gain-of-function mutation of Wnt signaling. We find that the dramatic proliferative effect that Notch signals have on early intestinal precursors requires normal Wnt signaling, whereas its influence on intestinal differentiation appears independent of Wnt. Analogous experiments in Drosophila demonstrate that the synergistic effects of Notch and Wnt are valid across species. We also demonstrate a striking synergy between Notch and Wnt signals that results in inducing the formation of intestinal adenomas, particularly in the colon, a region rarely affected in available mouse tumor models, but the primary target organ in human patients. These studies thus reveal a previously unknown oncogenic potential of Notch signaling in colorectal tumorigenesis that, significantly, is supported by the analysis of human tumors. Importantly, our experimental evidence raises the possibility that Notch activation might be an essential initial event triggering colorectal cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
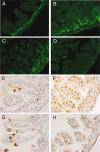
Notch activation is not sufficient to restore proliferation in Tcf4−/− mice. Paraffin longitudinal sections of neonatal small intestine from wild-type (A and E), vilCre/Nic (B and F), Tcf4−/− (C and G) and vilCre/Nic;Tcf4−/− (D and H) mice stained with the proliferation marker Ki67 in A–D and with an antibody anti-Hes1 in E–H. The increase in cell proliferation induced by Notch activation (green cells in B) is not visible in the absence of Tcf4 (D). The Notch target Hes1 is strongly induced in vilCre/Nic mice and in vilCre/Nic;Tcf4−/− mice (brown nuclei in F and H), indicating that Notch signaling is activated in these transgenic mice. Goblet cells show nonspecific staining with this polyclonal antibody (E and G). [Scale bar, 80 μm (A–D) and 50 μm (E–H).]
Fig. 2.
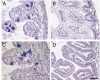
Notch impairs goblet cell differentiation independently of Tcf4. Paraffin longitudinal sections of neonatal small intestine from wild-type (A), vilCre/Nic (B), Tcf4−/− (C), and vilCre/Nic;Tcf4−/− (D) mice stained with Alcian blue to reveal differentiated goblet cells are shown. The light blue cells evident in A and C are absent in the presence of the activated Notch transgene (B), even in a Tcf4−/− background (D). (Scale bar, 50 μm.)
Fig. 3.
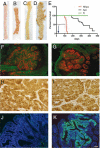
Notch and Wnt signals show a synergistic impact on intestinal tumorigenesis. (A–D) Small intestine (A and B) and colon (C and D) of N/Apc mice (B and D) present a high number of macroscopically visible lesions compared with control tissues (A and C). (E) Kaplan–Meier survival of N/Apc mice and their single transgenic littermates over a period of 437 days. The N/Apc compound mice (in red) are all dead by 4 months, whereas the Apc single-mutant mice (in black) start dying at ≈6 months of age, and the N mice (in green) present a normal lifespan. (F–K) Thin sections of intestinal adenomas from Apc+/− (F, H and J) and N/Apc (G, I, and K) mice. (F and G) Tissues are stained with the proliferation marker Ki67 (in red) and an antibody recognizing the villin protein (in green). (H and I) Antibody identifying β-catenin reveals cytoplasmic and nuclear localization of β-catenin in both tumors, indicative of active Wnt signaling. (J and K) Anti-GFP staining reveals the presence of the Notch-ires-GFP transgene in N/Apc tumors (K). [Scale bar, 1 cm (A–D), 50 μm (F, G, J, and K), and 25 μm (H and I).]
Fig. 4.
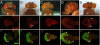
Synergy of Notch and Wingless in Drosophila. Adult eyes (Top) and corresponding larval eye imaginal discs of Drosophila in different genetic backgrounds (see Results for detailed genotypes), stained for BrdU (in red, Middle) to indicate cells in S phase and elav (in green, Bottom) a marker of neuronal differentiation. (B′ and E′) White arrows in indicate ectopic proliferation in the eye imaginal discs. (Bottom) White arrowheads indicate the positions of the morphogenetic furrow. (A–A″) Wild-type animals. (B–B″) Nic-expressing flies show a large-eye phenotype induced by Notch activation. Abnormal, ectopic BrdU incorporation is indicated by the arrow in B′, whereas neuronal differentiation is unaffected in B″. This phenotype is suppressed by expression of dominant-negative Pangolin, the Drosophila homolog of Tcf4. Flies expressing Nic and dominant-negative Pangolin present adult eye morphology, BrdU incorporation, and elav pattern of expression undistinguishable from wild-type flies (compare A–A″ with C–C″). In contrast, the expression of a gain-of-function wingless allele in these flies dramatically enhances the Nic phenotype (compare B–B″ with D–D″). (E–E″) A similar synergy is also observed in flies expressing Nic and harboring a loss-of-function mutation of the Wnt antagonist Apc.
Fig. 5.
The Notch target Hes1 is highly expressed in mouse and human adenomas but not in carcinomas. (A–D) Paraffin longitudinal sections of normal small intestine (A and B) and tumor tissues (C and D) from Apc+/− (A and C) and N/Apc (B and D) mice, stained with a rabbit antibody recognizing the Hes1 protein. Hes1 is found exclusively in the crypts of adult normal intestine (A), whereas in N/Apc mice its expression correlates with the mosaic expression of the Nic transgene (B). In mouse adenomas, Hes1 is strongly expressed both in N/Apc mice (D) and in Apc+/− control mice (C). Goblet cells present nonspecific staining with this antibody in A. (E–J) Paraffin representative sections of human specimens of normal colon (E and H), sporadic and FAP low-grade colon adenomas (F and I, respectively), and sporadic and FAP adenocarcinomas (G and J, respectively) stained with a rat anti-Hes1 antibody. Hes1 is readily detectable in the nuclei of both normal and dysplastic human colonic crypts (E, F, H, and I), but it is not expressed in human colon adenocarcinomas (G and J). [Scale bar, 100 μm (A and B) and 50 μm (C–J).]
Fig. 6.
Notch signaling is active in mouse and human tumors. (A) Real-time qPCR was used to quantify the expression levels of Hes1 mRNA in mouse samples. Hes1 expression is elevated in adenomas derived from the small intestine of Apc+/− mice to levels comparable with those found in N/Apc mice (compare the bar of Apc+/− adenomas with the bars corresponding to N/Apc normal intestine and adenomas). Expression of Hes1 in the normal intestine of Apc+/− mice is restricted to the crypt proliferative compartment, as illustrated in Fig. 5_A_. The standard deviation represents the variability between at least 5 different mice. Each sample was normalized to β2-microglobulin. (B–E) Real-time qPCR reveals that the Notch targets Hes1 (B) and HeyL (C) and the Notch ligand Jagged2 (E) are significantly up-regulated in human adenomas (n = 7) compared with human adenocarcinomas (n = 9). The levels of Notch1 mRNA (D) are also elevated in adenomas versus carcinomas, albeit to a lesser extent. The black horizontal lines represent the median value for each group of individual tumors. Each sample was normalized to β-actin (black bars), hypoxyanthine-guanine phosphoribosyltransferase (dark gray bars), and glyceraldehyde-3-phosphate dehydrogenase (light gray bars). (F) Schematic diagram illustrating the synergy of Notch and Wnt signals during intestinal tumor initiation. Activation of Notch signaling may occur before (Model 1), after (Model 2), or concomitantly (Model 3) with the occurrence of Apc mutations leading to constitutive activation of the Wnt cascade. Our results, however, favor Model 1 because in mice where Notch activation precedes LOH for Apc (N/Apc mice) we observe ≈20-fold more adenomas than in Apc+/− mice.
Similar articles
- Interplay between VHL/HIF1alpha and Wnt/beta-catenin pathways during colorectal tumorigenesis.
Giles RH, Lolkema MP, Snijckers CM, Belderbos M, van der Groep P, Mans DA, van Beest M, van Noort M, Goldschmeding R, van Diest PJ, Clevers H, Voest EE. Giles RH, et al. Oncogene. 2006 May 18;25(21):3065-70. doi: 10.1038/sj.onc.1209330. Oncogene. 2006. PMID: 16407833 - BCL9-2 promotes early stages of intestinal tumor progression.
Brembeck FH, Wiese M, Zatula N, Grigoryan T, Dai Y, Fritzmann J, Birchmeier W. Brembeck FH, et al. Gastroenterology. 2011 Oct;141(4):1359-70, 1370.e1-3. doi: 10.1053/j.gastro.2011.06.039. Epub 2011 Jun 23. Gastroenterology. 2011. PMID: 21703997 - Tripartite interactions between Wnt signaling, Notch and Myb for stem/progenitor cell functions during intestinal tumorigenesis.
Germann M, Xu H, Malaterre J, Sampurno S, Huyghe M, Cheasley D, Fre S, Ramsay RG. Germann M, et al. Stem Cell Res. 2014 Nov;13(3 Pt A):355-66. doi: 10.1016/j.scr.2014.08.002. Epub 2014 Sep 28. Stem Cell Res. 2014. PMID: 25290188 - Notch signaling in intestinal homeostasis across species: the cases of Drosophila, Zebrafish and the mouse.
Fre S, Bardin A, Robine S, Louvard D. Fre S, et al. Exp Cell Res. 2011 Nov 15;317(19):2740-7. doi: 10.1016/j.yexcr.2011.06.012. Epub 2011 Jun 30. Exp Cell Res. 2011. PMID: 21745469 Review. - Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision.
Nakamura T, Tsuchiya K, Watanabe M. Nakamura T, et al. J Gastroenterol. 2007 Sep;42(9):705-10. doi: 10.1007/s00535-007-2087-z. Epub 2007 Sep 25. J Gastroenterol. 2007. PMID: 17876539 Review.
Cited by
- Interconnection of CD133 Stem Cell Marker with Autophagy and Apoptosis in Colorectal Cancer.
Sipos F, Műzes G. Sipos F, et al. Int J Mol Sci. 2024 Oct 18;25(20):11201. doi: 10.3390/ijms252011201. Int J Mol Sci. 2024. PMID: 39456981 Free PMC article. Review. - Conserved Signaling Pathways in the Ciona robusta Gut.
Gerdol M, Greco S, Marino R, Locascio A, Plateroti M, Sirakov M. Gerdol M, et al. Int J Mol Sci. 2024 Jul 18;25(14):7846. doi: 10.3390/ijms25147846. Int J Mol Sci. 2024. PMID: 39063090 Free PMC article. - A state-of-the-art systematic review of cancer in hidradenitis suppurativa.
Abu Rached N, Rüth J, Gambichler T, Ocker L, Bechara FG. Abu Rached N, et al. Ann Med. 2024 Dec;56(1):2382372. doi: 10.1080/07853890.2024.2382372. Epub 2024 Jul 24. Ann Med. 2024. PMID: 39046819 Free PMC article. - Evaluation of in vitro and in vivo anticancer activities of potassium koetjapate: a solubility improved formulation of koetjapic acid against human colon cancer.
Jafari SF, Keshavarzi M, AbdulMajid AM, Al-Suede FSR, Asif M, Ahamed MBK, Khan MSS, Hassan LAE, Majid ASA, Naseri M. Jafari SF, et al. Res Pharm Sci. 2024 Apr 1;19(2):203-216. doi: 10.4103/RPS.RPS_247_22. eCollection 2024 Apr. Res Pharm Sci. 2024. PMID: 39035582 Free PMC article. - Crosstalk between MicroRNAs and Oxidative Stress in Coeliac Disease.
Pelizzaro F, Cardin R, Sarasini G, Minotto M, Carlotto C, Fassan M, Palo M, Farinati F, Zingone F. Pelizzaro F, et al. Inflamm Intest Dis. 2024 Jan 24;9(1):11-21. doi: 10.1159/000536107. eCollection 2024 Jan-Dec. Inflamm Intest Dis. 2024. PMID: 38298886 Free PMC article. Review.
References
- Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: Stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. - PubMed
- Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. - PubMed
- Andreu P, et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–1451. - PubMed
- van Es JH, et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA098402/CA/NCI NIH HHS/United States
- R37 NS026084/NS/NINDS NIH HHS/United States
- R01 NS026084/NS/NINDS NIH HHS/United States
- R01 CA098402/CA/NCI NIH HHS/United States
- NS26084/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases