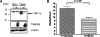X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas - PubMed (original) (raw)
X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas
Lorenzo Romero-Ramirez et al. Transl Oncol. 2009 Mar.
Abstract
Purpose: Tumors encounter endoplasmic reticulum stress during tumor growth and activate an adaptive pathway known as the unfolded protein response (UPR). Because this pathway is induced by the tumor microenvironment, it is a promising target for cancer therapy. We have previously demonstrated that X-box binding protein 1 (XBP-1), a key regulator of the UPR, was required for survival under hypoxia and critical for tumor growth in tumor xenografts. In this study, we investigated the role of XBP-1 in regulating tumor angiogenesis.
Methods: We used an intradermal angiogenesis model to quantify the effect of XBP-1 on angiogenesis. We also used a human tumor xenograft model to assay for tumor growth delay. We determined vascular endothelial growth factor (VEGF) expression by quantitative polymerase chain reaction and ELISA. Finally, we stained human pancreatic adenocarcinoma specimens for XBP-1 expression and correlated the expression pattern of XBP-1 with CD31 (endothelial cell marker) expression.
Results: We demonstrated that XBP-1 is essential for angiogenesis during early tumor growth. Inhibiting XBP-1 expression by short-hairpin RNA sequence specific for XBP-1 reduced blood vessel formation in tumors from mouse embryonic fibroblast cells and human fibrosarcoma tumor cells (HT1080). Expressing a dominant-negative form of IRE1alpha also reduced blood vessel formation in tumors. Moreover, expression of spliced XBP-1 (XBP-1s) restored angiogenesis in IRE1alpha dominant-negative expressing cells. We further demonstrated that XBP-1-mediated angiogenesis does not depend on VEGF.
Conclusions: We propose that the IRE1alpha-XBP-1 branch of the UPR modulates a complex proangiogenic, VEGF-independent response that depends on signals received from the tumor microenvironment.
Figures
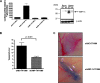
Figure 1
XBP-1 is essential for tumor angiogenesis in MEF cells. (A) Reduction of XBP-1 expression by a short-hairpin specific sequence was verified by qPCR for total XBP-1 messenger. (B) Tumor growth data from XBP-1 control MEF cells (shControl MEF) and XBP-1 shRNA-expressing cells (shXBP-1 MEF). Each SCID mouse was implanted subcutaneously with two tumors consisting of 2 x 106 shControl MEF cells in one flank and 2 x 106 shXBP-1 MEF cells in the contralateral flank. Error bars, SD of the mean from four tumors. (C) Angiogenesis assay in vivo for XBP-1 control MEF cells (shControl MEF) and XBP-1 shRNA-expressing cells (shXBP-1 MEF). SCID mice were intradermally implanted with 1 x 105 MEF cells. The number of vessels growing into the tumor was scored 3 and 6 days after implantation with each observer blinded to the treatment condition. Error bars, SE of the mean from at least six tumors. Statistical significance was determined using a 2-tailed t test.
Figure 2
XBP-1 is essential for tumor angiogenesis in HT1080 cells. Reduction of XBP-1 expression by a short-hairpin specific sequence was verified by (A) UPRE reporter assay (left panel) and Western blot analysis for spliced XBP-1 (XBP-1s-specific antibody, right panel). Cells were exposed to 8 hours of tunicamycin (5 _µ_g/ml for 8 hours) for the reporter assays and 24 hours of hypoxia (O2 < 0.02%) for the Western blot analysis to induce XBP-1 splicing. Expression of β-actin is included as a loading control. (B) Angiogenesis assay in vivo for XBP-1 scramble control HT1080 cells (shSc-HT1080) and XBP-1 shRNA-expressing cells (shXBP-1HT1080). SCID mice were intradermally implanted with 2 x 105 HT1080 cells. The number of vessels growing into the tumor was scored 7 days after implantation. Each observer quantitated the number of blood vessels independently and was blinded to the treatment condition. Error bars, SE of the mean from at least 10 tumors. Statistical significance was determined using a 2-tailed t test. (C) Representative photomicrographs of the tumors from angiogenesis assays. As shown in the bottom panel, there are significantly fewer capillaries growing into the HT1080 tumors with reduced XBP-1 expression (sh-XBP-1HT1080) compared with the scrambled shRNA control-expressing cells (top panel, shSCHT1080).
Figure 3
A dominant-negative form of IRE1α inhibits angiogenesis in HT1080 cells. (A) Western blot for spliced XBP-1 (XBP-1s), Flagtagged IRE1α dominant-negative protein (IΔC), and β-actin (as loading control). Cells were treated with 24 hours of hypoxia (O2 < 0.02%) to induce XBP-1 splicing. There was no XBP-1 splicing in the IRE1α dominant-negative expressing cells. (B) Angiogenesis assay in vivo for empty vector control cells (HT1080-pBabe) and flag-tagged IRE1α dominant-negative expressing cells (HT1080-IΔC). Error bars, SE of the mean from 10 tumors. Statistical significance was determined using a 2-tailed t test.
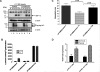
Figure 4
XBP-1 spliced form (XBP-1s) rescues tumor angiogenesis in IRE1α dominant-negative expressing cells. (A) Expression of XBP-1s and IRE1α dominant-negative in HT1080 cells was confirmed by Western blot analysis. Lanes 1 and 5 are HT1080 vector control cells. Lanes 2 and 6 are HT1080 cells transfected with a Flag-tagged IRE1α dominant-negative construct. Lanes 3 and 7 are HT1080 cells transfected with both Flag-tagged IRE1α dominant-negative construct and an XBP-1 overexpression construct. Lanes 4 and 8 are HT1080 cells transfected with XBP-1 overexpression construct. Tunicamycin (5 _µ_g/ml for 8 hours) was used to induce XBP-1 splicing. β-Actin was used as a loading control. Western blot analysis was carried out using XBP-1s-specific antibody (Biolegend). (B) XBP-1 reporter (UPRE-luciferase) assay in HT1080 cells expressing vector alone (vector-ϕ), IRE1α dominant-negative (vector-ΔC), or IRE1α dominant-negative and spliced XBP-1 (ΔCS). All cells were treated with tunicamycin (5 _µ_g/ml for 8 hours). Expression of IRE1α dominant-negative blocked transactivation of the UPRE-luciferase reporter during tunicamycin treatment. Inhibition of the UPRE-luciferase reporter could be reversed by overexpression of XBP-1s. (C) Angiogenesis assay for HT1080 cells expressing vector alone (vector-ϕ), IRE1α dominant-negative (ϕ-ΔC), or IRE1α dominant-negative and spliced XBP-1 (ΔCS). Error bars, SE of the mean from at least eight tumors. Statistical significance was determined using a 2-tailed t test. (D) VEGF expression by qPCR in HT1080 control cells (shSC-HT1080) or HT1080 cells inhibited in XBP-1 expression by shRNA (shXBP-1HT1080). There was no difference in VEGF expression at baseline or during hypoxia between these two cell lines.
Figure 5
VEGF mRNA expression is not regulated by XBP-1. (A) The top panel shows strong CD31 staining of endothelial cells in a human pancreatic adenocarcinoma. The bottom panel is an adjacent pancreatic tumor section showing strong XBP-1s expression. (B) Thirty-two consecutive pancreatic tumor resection specimens were stained for CD31 and XBP-1s expression. We found a strong correlation between XBP-1s staining and CD31 staining (P = .016) suggesting that XBP-1s is a clinically relevant to angiogenesis in pancreatic cancer.
Similar articles
- Reduced response of IRE1α/Xbp-1 signaling pathway to bortezomib contributes to drug resistance in multiple myeloma cells.
Xu X, Liu J, Huang B, Chen M, Yuan S, Li X, Li J. Xu X, et al. Tumori. 2017 May 12;103(3):261-267. doi: 10.5301/tj.5000554. Epub 2016 Sep 7. Tumori. 2017. PMID: 27647225 - XBP-1, a key regulator of unfolded protein response, activates transcription of IGF1 and Akt phosphorylation in zebrafish embryonic cell line.
Hu MC, Gong HY, Lin GH, Hu SY, Chen MH, Huang SJ, Liao CF, Wu JL. Hu MC, et al. Biochem Biophys Res Commun. 2007 Aug 3;359(3):778-83. doi: 10.1016/j.bbrc.2007.05.183. Epub 2007 Jun 4. Biochem Biophys Res Commun. 2007. PMID: 17560942 - Impaired IRE1α/XBP-1 pathway associated to DNA methylation might contribute to salivary gland dysfunction in Sjögren's syndrome patients.
Sepúlveda D, Barrera MJ, Castro I, Aguilera S, Carvajal P, Lagos C, González S, Albornoz N, Bahamondes V, Quest AFG, Urzúa U, Molina C, Leyton C, Hermoso MA, González MJ. Sepúlveda D, et al. Rheumatology (Oxford). 2018 Jun 1;57(6):1021-1032. doi: 10.1093/rheumatology/key021. Rheumatology (Oxford). 2018. PMID: 29534223 - XBP-1, a Cellular Target for the Development of Novel Anti-viral Strategies.
Ong HK, Soo BPC, Chu KL, Chao SH. Ong HK, et al. Curr Protein Pept Sci. 2018;19(2):145-154. doi: 10.2174/1389203718666170911144812. Curr Protein Pept Sci. 2018. PMID: 28901250 Review. - The role of X-box binding protein-1 in tumorigenicity.
Shajahan AN, Riggins RB, Clarke R. Shajahan AN, et al. Drug News Perspect. 2009 Jun;22(5):241-6. doi: 10.1358/dnp.2009.22.5.1378631. Drug News Perspect. 2009. PMID: 19609461 Free PMC article. Review.
Cited by
- The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies.
Luo B, Lee AS. Luo B, et al. Oncogene. 2013 Feb 14;32(7):805-18. doi: 10.1038/onc.2012.130. Epub 2012 Apr 16. Oncogene. 2013. PMID: 22508478 Free PMC article. Review. - The evolving paradigm of cell-nonautonomous UPR-based regulation of immunity by cancer cells.
Zanetti M, Rodvold JJ, Mahadevan NR. Zanetti M, et al. Oncogene. 2016 Jan 21;35(3):269-78. doi: 10.1038/onc.2015.108. Epub 2015 Apr 20. Oncogene. 2016. PMID: 25893303 Review. - The Emerging Roles of Endoplasmic Reticulum Stress in Balancing Immunity and Tolerance in Health and Diseases: Mechanisms and Opportunities.
Li A, Song NJ, Riesenberg BP, Li Z. Li A, et al. Front Immunol. 2020 Feb 11;10:3154. doi: 10.3389/fimmu.2019.03154. eCollection 2019. Front Immunol. 2020. PMID: 32117210 Free PMC article. Review. - Extensive pancreas regeneration following acinar-specific disruption of Xbp1 in mice.
Hess DA, Humphrey SE, Ishibashi J, Damsz B, Lee AH, Glimcher LH, Konieczny SF. Hess DA, et al. Gastroenterology. 2011 Oct;141(4):1463-72. doi: 10.1053/j.gastro.2011.06.045. Epub 2011 Jun 24. Gastroenterology. 2011. PMID: 21704586 Free PMC article. - Overexpression of X-Box Binding Protein 1 (XBP1) Correlates to Poor Prognosis and Up-Regulation of PI3K/mTOR in Human Osteosarcoma.
Yang J, Cheng D, Zhou S, Zhu B, Hu T, Yang Q. Yang J, et al. Int J Mol Sci. 2015 Dec 2;16(12):28635-46. doi: 10.3390/ijms161226123. Int J Mol Sci. 2015. PMID: 26633383 Free PMC article.
References
- Feldman DE, Chauhan V, Koong AC. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol Cancer Res. 2005;3:597–605. - PubMed
- Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–977. - PubMed
- Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–5947. - PubMed
- Chen Y, Feldman DE, Deng C, Brown JA, De Giacomo AF, Gaw AF, Shi G, Le QT, Brown JM, Koong AC. Identification of mitogen-activated protein kinase signaling pathways that confer resistance to endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol Cancer Res. 2005;3:669–677. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials