Conformational changes and protein stability of the pro-apoptotic protein Bax - PubMed (original) (raw)
Conformational changes and protein stability of the pro-apoptotic protein Bax
Stephanie Bleicken et al. J Bioenerg Biomembr. 2009 Feb.
Abstract
Pro-apoptotic Bax is a soluble and monomeric protein under normal physiological conditions. Upon its activation substantial structural rearrangements occur: The protein inserts into the mitochondrial outer membrane and forms higher molecular weight oligomers. Subsequently, the cells can undergo apoptosis. In our studies, we focused on the structural rearrangements of Bax during oligomerization and on the protein stability. Both protein conformations exhibit high stability against thermal denaturation, chemically induced unfolding and proteolytic processing. The oligomeric protein is stable up to 90 degrees C as well as in solutions of 8 M urea or 6 M guanidinium hydrochloride. Helix 9 appears accessible in the monomer but hidden in the oligomer assessed by proteolysis. Tryptophan fluorescence indicates that the environment of the C-terminal protein half becomes more apolar upon oligomerization, whereas the loop region between helices 1 and 2 gets solvent exposed.
Figures
Fig. 1
Representation of conserved regions, and hydrophobicity in the Bax structure. The secondary as well as a cartoon of the ternary structure of Bax (PDB:1F16) are shown in a and b, respectively. Helices are colored according to the code: α1 yellow, α2 fawn, α3 orange, α4 pink, α5 light red, α6 dark red, α7 purple, α8 blue, α9 green. c Shows a representation of the sequence conservation of the Bax surface. Conserved amino acids were identified by ClustalW alignment of human Bax alpha and its orthologs of Xenopus laevis and Danio rerio and were highlighted in the published NMR structure of human Bax alpha (Suzuki et al. 2000). Amino acids identical in all sequences are shown in red. Substitutions which were classified as highly conserved residues are marked in orange and semi-conserved ones are displayed in yellow. d Demonstrates the hydrophobicity of the surface exposed amino acids (according to the hydropathy index by Kyte and Doolittle 1982). Acidic [E,D] and basic [K,R] amino acids are shown in red and blue, respectively. Hydrophobic amino acids [I,V,L,A,C,F,M] are shown in green and slightly hydrophobic residues [G,T,S,W] in light green. Others [Q,N,P,H,Y] are marked in grey. b–d Show two views of the molecule tilted by 180°
Fig. 2
Amino acid sequence alignment of orthologous Bax alpha representatives from mammals and vertebrates. Amino acids identical in all sequences are colored in red and labeled with asterisks. Substitutions of highly conserved residues are marked in orange with colons and of semi-conserved residues are displayed in yellow indicated with periods. The secondary structure (for color code see Fig. 1a) as well as the position of the BH domains of Bax are also shown
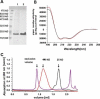
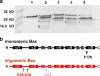
Fig. 3
Purification, folding and oligomerization of the Bax protein. The SDS gel shown in a demonstrates the purity of Bax: (1) after the chitin affinity chromatography (first purification step) (2) after anion exchange chromatography (second purification step). Molecular weight standard bands are indicated on the left margin. The calculated molecular weight of Bax is 21 kD. b CD spectra of monomeric (black) and oligomeric Bax in 0.5% DDM (red). c SEC analysis of monomeric and oligomeric Bax as well as monomeric Bax pre-incubated at 90 °C. Monomeric Bax is shown in black and eluted as a monomer with a small amount of dimers. Oligomeric Bax (in 0.5% DDM) is shown in red and monomeric Bax pre-incubated at 90 °C in purple. The arrows mark the exclusion volume as well as the elution maxima of ferritin (440 kD) and chymotrysiongen A (25 kD)
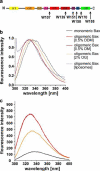
Fig. 4
Tryptophan fluorescence of Bax. The positions of the tryptophans in the secondary structure of Bax are highlighted by arrows and corresponding labels in a. The color code is the same as described for Fig. 1a. TF spectra (normalized to 1) of monomeric and oligomeric Bax in different detergents as well as Bax reconstituted in liposomes are shown in b. Not Normalized TF spectra are shown in c with the same color code as indicated in b
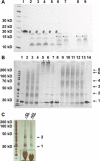
Fig. 5
Analysis of subtilisin treated Bax. Fragments of differently treated Bax samples after proteolysis with subtilisin are shown on a coomassie-stained 17% SDS gel in a. Untreated monomeric Bax was loaded onto lane 1, subtilisin treated monomeric Bax is shown in lane 2, oligomeric Bax (in 0.5% DDM) after proteolysis with subtilisin refers to lane 3. Monomeric and oligomeric (in 0.5% DM) Bax both pre-heated to 90 °C are presented in lane 4 and lane 5, respectively. Subsequent analysis of proteolytic products after heating revealed different fragments of Bax, which are labeled with asterisks for full length Bax (aa 1–192 and aa 1–191), number sign for aa 1–176, aa 1–174 and aa 1–173, degree symbol for aa 39–192, aa 47–192, aa 39–191 and aa 47–191, plus symbol aa 82–192 and aa 83–192 as well as section symbol for aa 1–39 and aa1– 46. In b the main cleavage sites in monomeric (black) and oligomeric (red) Bax are highlighted by arrows
Fig. 7
Properties of heat treated Bax. Monomeric and oligomeric Bax were incubated at different temperatures for 10 min, cooled on ice and treated with proteinase K for 1 h on ice (a) or cross linked (b). Cross link oligomers in liposomes are shown in c. Separation was done on 4–12% gradient SDS gels and analyzed. a Untreated monomeric Bax was loaded onto lane 1, proteinase K treated monomeric Bax pre-incubated at different temperatures were loaded onto lanes 2 through 7: lane 2 4 °C, lane 3 50 °C, lane 4 60 °C, lane 5 70 °C, lane 6 80 °C and lane 7 90 °C. In lane 8 and 9 oligomeric Bax (in 0.5% DM) was pre-incubated at different temperatures: lane 8 4 °C, lane 9 90 °C. Mass analysis of the proteolytic products after pre-heating revealed differently sized Bax fragments. The labeling is equal to that shown in Fig. 5a. b Monomeric and oligomeric Bax proteins cross-linked after pre-incubation at different temperatures: Lane 1 untreated monomeric Bax pre-incubated at 4 °C, lane 2 to 6: monomeric Bax after pre-incubation at different temperatures and cross-linking for 5 s: lane 2 4 °C, lane 3 50 °C, lane 4 70 °C, lane 5 80 °C, lane 6 90 °C, lane 7 monomeric Bax pre-incubated at 90 °C without cross-linking, lane 8 untreated oligomeric Bax (0.5% DDM) pre-incubated at 4 °C, lane 9–13 oligomeric Bax (0.5% DDM) pre-incubated at different temperatures and after cross-linking for 5 s, lane 9 4 °C, lane 10 50 °C, lane 11 70 °C, lane 12 80 °C, lane 13 90 °C, lane 14 oligomeric Bax (0.5% DDM) pre-incubated at 90 °C without cross-linking. The main visible band and the number of cross linked monomers per band are indicated by arrows and numbers on the right margin. The asterisk indicates aggregates or big oligomers that did not enter the gel. c Demonstration of a silver stained SDS-gel of reconstituted Bax oligomers (without heat treatment) before and after cross-linking. The arrows indicates monomers and dimers. The bands framed by the black box are not protein but lipid bands
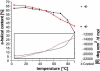
Fig. 6
Melting behavior of heat treated monomeric and oligomeric Bax. The thermal stability of monomeric (black) and oligomeric (red) Bax is shown. The upper panel depicts the decrease of the alpha helical content of Bax during the temperature increase, estimated from CD spectra taken at different temperatures. The alpha helical content of Bax heated to 90 °C and recooled to 4 °C is highlighted by arrows. The lower inset panel indicates the decrease of the ellipticity at 222 nm measured during the whole temperature increase
Fig. 8
TF of monomeric and oligomeric Bax after treatment with denaturing agents. Treatment with urea is shown in a and treatment with guanidinium hydrochloride in b. The data were normalized and maximal TF emission was set to 1
Similar articles
- Synthetic Antibodies Inhibit Bcl-2-associated X Protein (BAX) through Blockade of the N-terminal Activation Site.
Uchime O, Dai Z, Biris N, Lee D, Sidhu SS, Li S, Lai JR, Gavathiotis E. Uchime O, et al. J Biol Chem. 2016 Jan 1;291(1):89-102. doi: 10.1074/jbc.M115.680918. Epub 2015 Nov 12. J Biol Chem. 2016. PMID: 26565029 Free PMC article. - Inhibition of Pro-apoptotic BAX by a noncanonical interaction mechanism.
Barclay LA, Wales TE, Garner TP, Wachter F, Lee S, Guerra RM, Stewart ML, Braun CR, Bird GH, Gavathiotis E, Engen JR, Walensky LD. Barclay LA, et al. Mol Cell. 2015 Mar 5;57(5):873-886. doi: 10.1016/j.molcel.2015.01.014. Epub 2015 Feb 12. Mol Cell. 2015. PMID: 25684204 Free PMC article. - Structural biology of the Bcl-2 family of proteins.
Petros AM, Olejniczak ET, Fesik SW. Petros AM, et al. Biochim Biophys Acta. 2004 Mar 1;1644(2-3):83-94. doi: 10.1016/j.bbamcr.2003.08.012. Biochim Biophys Acta. 2004. PMID: 14996493 Review. - Effect of pH on stability, conformation, and chaperone activity of erythroid & non-erythroid spectrin.
Bose D, Patra M, Chakrabarti A. Bose D, et al. Biochim Biophys Acta Proteins Proteom. 2017 Jun;1865(6):694-702. doi: 10.1016/j.bbapap.2017.03.012. Epub 2017 Apr 2. Biochim Biophys Acta Proteins Proteom. 2017. PMID: 28373029 - Discoveries and controversies in BCL-2 protein-mediated apoptosis.
Zheng JH, Viacava Follis A, Kriwacki RW, Moldoveanu T. Zheng JH, et al. FEBS J. 2016 Jul;283(14):2690-700. doi: 10.1111/febs.13527. Epub 2015 Oct 27. FEBS J. 2016. PMID: 26411300 Review.
Cited by
- ROCK1 plays an essential role in the transition from cardiac hypertrophy to failure in mice.
Shi J, Zhang YW, Yang Y, Zhang L, Wei L. Shi J, et al. J Mol Cell Cardiol. 2010 Nov;49(5):819-28. doi: 10.1016/j.yjmcc.2010.08.008. Epub 2010 Aug 13. J Mol Cell Cardiol. 2010. PMID: 20709073 Free PMC article. - Proapoptotic Bax and Bak proteins form stable protein-permeable pores of tunable size.
Bleicken S, Landeta O, Landajuela A, Basañez G, García-Sáez AJ. Bleicken S, et al. J Biol Chem. 2013 Nov 15;288(46):33241-52. doi: 10.1074/jbc.M113.512087. Epub 2013 Oct 7. J Biol Chem. 2013. PMID: 24100034 Free PMC article. - High-resolution analysis of the conformational transition of pro-apoptotic Bak at the lipid membrane.
Sperl LE, Rührnößl F, Schiller A, Haslbeck M, Hagn F. Sperl LE, et al. EMBO J. 2021 Oct 18;40(20):e107159. doi: 10.15252/embj.2020107159. Epub 2021 Sep 15. EMBO J. 2021. PMID: 34523144 Free PMC article. - SETMAR isoforms in glioblastoma: A matter of protein stability.
Dussaussois-Montagne A, Jaillet J, Babin L, Verrelle P, Karayan-Tapon L, Renault S, Rousselot-Denis C, Zemmoura I, Augé-Gouillou C. Dussaussois-Montagne A, et al. Oncotarget. 2017 Feb 7;8(6):9835-9848. doi: 10.18632/oncotarget.14218. Oncotarget. 2017. PMID: 28038463 Free PMC article. - Insights into the structural stability of Bax from molecular dynamics simulations at high temperatures.
Rosas-Trigueros JL, Correa-Basurto J, Benítez-Cardoza CG, Zamorano-Carrillo A. Rosas-Trigueros JL, et al. Protein Sci. 2011 Dec;20(12):2035-46. doi: 10.1002/pro.740. Epub 2011 Nov 1. Protein Sci. 2011. PMID: 21936009 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials