Hematopoietic and endothelial differentiation of human induced pluripotent stem cells - PubMed (original) (raw)
Hematopoietic and endothelial differentiation of human induced pluripotent stem cells
Kyung-Dal Choi et al. Stem Cells. 2009 Mar.
Abstract
Induced pluripotent stem cells (iPSCs) provide an unprecedented opportunity for modeling of human diseases in vitro, as well as for developing novel approaches for regenerative therapy based on immunologically compatible cells. In this study, we employed an OP9 differentiation system to characterize the hematopoietic and endothelial differentiation potential of seven human iPSC lines obtained from human fetal, neonatal, and adult fibroblasts through reprogramming with POU5F1, SOX2, NANOG, and LIN28 and compared it with the differentiation potential of five human embryonic stem cell lines (hESC, H1, H7, H9, H13, and H14). Similar to hESCs, all iPSCs generated CD34(+)CD43(+) hematopoietic progenitors and CD31(+)CD43(-) endothelial cells in coculture with OP9. When cultured in semisolid media in the presence of hematopoietic growth factors, iPSC-derived primitive blood cells formed all types of hematopoietic colonies, including GEMM colony-forming cells. Human induced pluripotent cells (hiPSCs)-derived CD43(+) cells could be separated into the following phenotypically defined subsets of primitive hematopoietic cells: CD43(+)CD235a(+)CD41a(+/-) (erythro-megakaryopoietic), lin(-)CD34(+)CD43(+)CD45(-) (multipotent), and lin(-)CD34(+)CD43(+)CD45(+) (myeloid-skewed) cells. Although we observed some variations in the efficiency of hematopoietic differentiation between different hiPSCs, the pattern of differentiation was very similar in all seven tested lines obtained through reprogramming of human fetal, neonatal, or adult fibroblasts with three or four genes. Although several issues remain to be resolved before iPSC-derived blood cells can be administered to humans for therapeutic purposes, patient-specific iPSCs can already be used for characterization of mechanisms of blood diseases and for identification of molecules that can correct affected genetic networks.
Figures
Figure 1
Flow cytometric analysis of CD34+ and CD43+ subsets generated in OP9 coculture from (A) hiPSCs (IPS(SK46)-M4-10) and (B) hESCs (H1). There are striking similarities between the subsets of hematopoietic cells developed from these two types of pluripotent cells. Representative analysis of 5 independent experiments is shown.
Figure 2
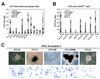
CFC potential of hESC and hiPSC lines. (A) CFC potential of total cells obtained at day 8 of OP9 coculture. (B) CFC potential of CD43+ and CD43− MACS sorted cells. Results are mean±SE of 3–5 independent experiments. (C) Morphology (upper row, bars are 100 µm) and Wright-stained cytospins (lower row, bars are 50 µm) of CFCs obtained from (SK46)-M4-10 hiPSCs.
Figure 3
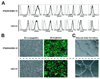
Phenotype and function of hiPSC- and hESC-derived endothelial cells. Isolated CD31+CD43− cells were cultured for 7 days in endothelial conditions. (A) Expression of markers of endothelial cells by flow cytometry. (B) Typical morphology of endothelial cell monolayer formed by CD31+CD43− cells (left panels) and immunofluorescent staining of monolayer for VE-cadherin (right panel); cell nuclei visualized by DAPI staining. (C) Vascular tube formation by expanded endothelial cells. All bars are 100µm. Representative experiment of 3 independent experiments is shown.
Figure 4
Flow cytometric analysis of phenotype of hiPSC- (A) and hESC-derived (B) CD34+CD43+CD45− and CD34+CD43+CD45+ primitive hematopoietic cells. Plots show isotype control (open gray) and specific antibody (open black) histograms. The representative experiment of 3–5 independent experiments is shown.
Figure 5
Kinetic analysis of hematopoietic and endothelial development from pluripotent stem cells in OP9 coculture (days 6–12 of differentiation). (A) Percentages of CD43+ hematopoietic and CD31+CD43− endothelial cells within live human (TRA-1-85+7-AAD−) cells (upper row), CD235a/CD41a+, CD235a/CD41a−CD45− and CD235a/CD41a−CD45+ within gated CD43+ cells (middle row) and CD14− cells within gated CD45+ cells (lower row) are shown. (B) Expression of myeloid lineage markers on hiPSC- and hESC-derived CD45+ cells following differentiation in OP9 cocluture.
Similar articles
- Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures.
Vodyanik MA, Thomson JA, Slukvin II. Vodyanik MA, et al. Blood. 2006 Sep 15;108(6):2095-105. doi: 10.1182/blood-2006-02-003327. Epub 2006 Jun 6. Blood. 2006. PMID: 16757688 Free PMC article. - Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors.
Choi KD, Vodyanik MA, Slukvin II. Choi KD, et al. J Clin Invest. 2009 Sep;119(9):2818-29. doi: 10.1172/JCI38591. Epub 2009 Aug 10. J Clin Invest. 2009. PMID: 19726877 Free PMC article. - Role of SOX17 in hematopoietic development from human embryonic stem cells.
Nakajima-Takagi Y, Osawa M, Oshima M, Takagi H, Miyagi S, Endoh M, Endo TA, Takayama N, Eto K, Toyoda T, Koseki H, Nakauchi H, Iwama A. Nakajima-Takagi Y, et al. Blood. 2013 Jan 17;121(3):447-58. doi: 10.1182/blood-2012-05-431403. Epub 2012 Nov 20. Blood. 2013. PMID: 23169777 - Evolution of induced pluripotent stem cell technology.
Zhou H, Ding S. Zhou H, et al. Curr Opin Hematol. 2010 Jul;17(4):276-80. doi: 10.1097/MOH.0b013e328339f2ee. Curr Opin Hematol. 2010. PMID: 20442654 Review. - Deciphering the hierarchy of angiohematopoietic progenitors from human pluripotent stem cells.
Slukvin II. Slukvin II. Cell Cycle. 2013 Mar 1;12(5):720-7. doi: 10.4161/cc.23823. Epub 2013 Feb 6. Cell Cycle. 2013. PMID: 23388453 Free PMC article. Review.
Cited by
- Beyond CAR-T: The rise of CAR-NK cell therapy in asthma immunotherapy.
Mohammad Taheri M, Javan F, Poudineh M, Athari SS. Mohammad Taheri M, et al. J Transl Med. 2024 Aug 5;22(1):736. doi: 10.1186/s12967-024-05534-8. J Transl Med. 2024. PMID: 39103889 Free PMC article. Review. - Resident vascular Sca1+ progenitors differentiate into endothelial cells in vascular remodeling via miR-145-5p/ERG signaling pathway.
Ying Z, Lyu L, Xu X, Wen Z, Xue J, Chen M, Li Z, Jiang L, Chen T. Ying Z, et al. iScience. 2024 May 22;27(6):110080. doi: 10.1016/j.isci.2024.110080. eCollection 2024 Jun 21. iScience. 2024. PMID: 38883819 Free PMC article. - Progresses in overcoming the limitations of in vitro erythropoiesis using human induced pluripotent stem cells.
Ju H, Sohn Y, Nam Y, Rim YA. Ju H, et al. Stem Cell Res Ther. 2024 May 15;15(1):142. doi: 10.1186/s13287-024-03754-9. Stem Cell Res Ther. 2024. PMID: 38750578 Free PMC article. Review. - Tissue-Engineered Microvessels: A Review of Current Engineering Strategies and Applications.
Zhao N, Pessell AF, Zhu N, Searson PC. Zhao N, et al. Adv Healthc Mater. 2024 Aug;13(21):e2303419. doi: 10.1002/adhm.202303419. Epub 2024 May 9. Adv Healthc Mater. 2024. PMID: 38686434 Review. - Prostate cancer research: tools, cell types, and molecular targets.
Liu AY. Liu AY. Front Oncol. 2024 Mar 26;14:1321694. doi: 10.3389/fonc.2024.1321694. eCollection 2024. Front Oncol. 2024. PMID: 38595814 Free PMC article. Review.
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. Epub 2006 Aug 2010. - PubMed
- Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. - PubMed
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. Epub 2007 Jun 2006. - PubMed
- Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. Epub 2007 Jun 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL081962-02/HL/NHLBI NIH HHS/United States
- P51 RR000167/RR/NCRR NIH HHS/United States
- R01 HL081962/HL/NHLBI NIH HHS/United States
- P51 RR000167-485069/RR/NCRR NIH HHS/United States
- R21 HL085223-02/HL/NHLBI NIH HHS/United States
- R21 HL085223/HL/NHLBI NIH HHS/United States
- HL081962/HL/NHLBI NIH HHS/United States
- HL085223/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous