Redox regulation of ephrin/integrin cross-talk - PubMed (original) (raw)
. 2007 Jan-Mar;1(1):33-42.
Epub 2007 Jan 29.
Affiliations
- PMID: 19262085
- PMCID: PMC2633678
Redox regulation of ephrin/integrin cross-talk
Francesca Buricchi et al. Cell Adh Migr. 2007 Jan-Mar.
Abstract
Interactions linking the Eph receptor tyrosine kinase and ephrin ligands transduce short-range repulsive signals regulating several motile biological processes including axon path-finding, angiogenesis and tumor growth. These ephrin-induced effects are believed to be mediated by alterations in actin dynamics and cytoskeleton reorganization. The members of the small Rho GTPase family elicit various effects on actin structures and are probably involved in Eph receptor-induced actin modulation. In particular, some ephrin ligands lead to a decrease in integrin-mediated cell adhesion and spread. Here we show that the ability of ephrinA1 to inhibit cell adhesion and spreading in prostatic carcinoma cells is strictly dependent on the decrease in the activity of the small GTPase Rac1. Given the recognized role of Rac-driven redox signaling for integrin function, reported to play an essential role in focal adhesion formation and in the overall organization of actin cytoskeleton, we investigated the possible involvement of oxidants in ephrinA1/EphA2 signaling. We now provide evidence that Reactive Oxygen Species are an integration point of the ephrinA1/integrin interplay. We identify redox circuitry in which the ephrinA1-mediated inhibition of Rac1 leads to a negative regulation of integrin redox signaling affecting the activity of the tyrosine phosphatase LMW-PTP. The enzyme in turn actively dephosphorylates its substrate p190RhoGAP, finally leading to RhoA activation. Altogether our data suggest a redox-based Rac-dependent upregulation of Rho activity, concurring with the inhibitory effect elicited by ephrinA1 on integrin-mediated adhesion strength.
Figures
Figure 1
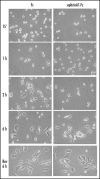
EphrinA1-Fc treatment inhibits adhesion and spreading of PC3 cells. Twenty-four hours after starvation, PC3 cells were detached and presuspended for 30 minutes in serum-free medium. Cells were stimulated with ephrinA1-Fc or Fc 1µg/ml and allowed to adhere to FN-coated dishes (10 µg/ml) for the indicated times and then were observed under a phase contrast microscope. After 6 hours of adhesion cells were detached with trypsinization and replated onto FN-coated dishes in serum-free medium; photographs were taken after 6 hours of adhesion.
Figure 2
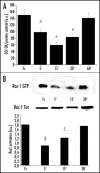
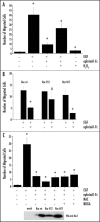
EphrinA1-induced decrease in intracellular hydrogen peroxide content is Rac-dependent: (A) ROS content measurement: 1 x 106 PC3 cells were serum-starved for 24 h and then stimulated with Fc or ephrinA1-Fc for the indicated times. Hydrogen peroxide production was evaluated with 2′,7′-dichlorofluorescein diacetate (DCF-DA). Five minutes before the end of ephrinA1-Fc stimulation, cells were treated with 5 µg/ml DCF-DA. After PBS washing, cells were lysed in 1 ml of RIPA buffer and analyzed immediately by fluorescence spectrophotometric analysis at 510 nm. Data were normalized on total protein content. Results are means ± SD of four independent experiments. #p < 0.005 vs Fc; *p < 0.001 vs Fc. (B) Rac activity after ephrinA1-Fc stimulation: 1 x 106 PC3 cells were treated as in A and Rac-GTP was analyzed by a pull-down assay performed with PAK-GST-agarose beads. The total amount of Rac-1 was then quantified by anti-Rac immunoblot. The mean ratio between the two values obtained from three independent experiments and SD are reported in the plot. §p < 0.01 vs Fc; ‡p < 0.05 vs Fc.
Figure 3
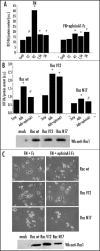
EphrinA1 inhibits integrin-dependent hydrogen peroxide generation. (A) Evaluation of hydrogen peroxide during adhesion in concomitance with ephrinA1-Fc stimulation. Twenty-four hours after starvation PC3 cells were detached, presuspended for 30 minutes in serum-free medium and kept in suspension or seeded onto fibronectin (FN)-coated dishes in the presence or not of 1 µg/ml ephrinA1-Fc for the indicated times. Hydrogen peroxide production was measured as in Figure 2. Results are means ± SD of four independent experiments. #p < 0.005 vs suspension; *p < 0.001 vs suspension (B) Rac-1 regulates ephrinA1-dependent inhibition of integrin-mediated hydrogen peroxide generation. Rac wt, Rac V12 (constitutive active mutant) and Rac N17 (dominant negative) were overexpressed in PC3 cells. After 24 hours of starvation, PC3 were detached, presuspended for 30 minutes in serum-free medium and kept in suspension or plated onto FN-coated dishes for 45 minutes in the presence or not of ephrinA1-Fc. ROS production was measured as in Figure 2. Means and SD of four independent experiments are indicated in the graph. #p < 0.005 vs suspension; *p < 0.001 vs suspension. Transfection efficiency of Rac mutants was evaluated by Western blot. (C) Rac-1 regulates ephrinA1-dependent inhibition of integrin-mediated cell adhesion. Rac wt, Rac V12 and Rac N17 expressing plasmids were overexpressed in PC3 cells. After 24 hours of starvation, the cells were detached, presuspended for 30 minutes in serum-free medium and plated onto FN-coated dishes in the presence or not of ephrinA1-Fc. Photographs were taken with a phase contrast microscope at the end of the adhesion time. Transfection efficiency of Rac mutants was evaluated by Western blot.
Figure 4
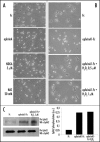
Antioxidant and H2O2 treatment influences ephrinA1-dependent inhibition of cell adhesion and spreading. (A) Effect of antioxidant treatment: 24 h after starvation PC3 cells have been detached and presuspended for 30 minutes in serum-free medium. Cells were then treated with 20 mM NAC and 5 µM NDGA or stimulated with ephrinA1-Fc and then allowed to adhere to FN-coated dishes for 45 minutes. Afterward the cells were observed under a phase contrast microscope (B) Effect of hydrogen peroxide treatment. Cells were treated as in A except that before adhesion they were treated with hydrogen peroxide at the indicated doses and/or with ephrinA1-Fc 1 µg/ml. After 45 minutes of adhesion, photographs were taken under a phase contrast microscope. (C) Activation of EphA2 induced by stimulation with ephrinA1-Fc or a mixture of ephrinA1-Fc plus hydrogen peroxide. In order to exclude that the effect of oxidant treatment could be due to ligand inactivation we stimulated PC3 cells with ephrinA1-Fc or with a previously prepared mixture of ephrinA1-Fc 1 µg/ml/H2O2 1 µM for 15 minutes. An IP with ephrinA1-Fc was performed followed by Western blot with anti-phosphotyrosine-specific antibodies. The membrane was then stripped and reprobed with anti-EphA2 specific antibodies for normalization.
Figure 5
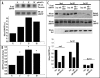
EphrinA1-induced inhibition of PC3 cell migration depends on the intracellular redox state. Cells were loaded into the upper compartment (5 x 105 cells in 500 µl) in serum-deprived growth medium, with or without 1 µg/ml of ephrinA1-Fc. The upper chambers were placed into 6-well culture dishes containing 1 ml of medium with 50 ng/ml human EGF as chemoattractant. After 24 h of incubation at 37°C, non-invading cells were removed mechanically using cotton swabs, and the micro-porous membrane containing the invaded cells was stained with DiffQuick solution. In the experiment reported (A) the cells were treated with H2O2 1 µM and (B) the cells were treated with NAC 20 mM or NDGA 5 µM. In the experiment reported (C) the cells were previously transfected with Rac wt, Rac V12 and Rac N17. Chemotaxis was evaluated by counting the cells that had migrated to the lower surface of the polycarbonate filters. For each filter the number of cells in ten randomly chosen fields was determined, and the average and SD were then calculated. #p < 0.005 vs control (untreated cells for experiment A and B and EGF-stimulated cells for experiment C); *p < 0.001 vs control (untreated cells for experiment A and B and EGF-stimulated cells for experiment C). Transfection efficiency of Rac mutants was evaluated by Western blot.
Figure 6
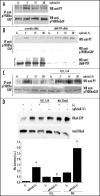
EphrinA1 stimulation influences the redox state of LMW-PTP. (A) Evaluation of reduced LMW-PTP during ephrinA1-Fc stimulation. PC3 cells after 24 hours of starvation were stimulated with ephrinA1-Fc 1 µg/ml for the indicated times. Cells were then lysed in the presence of 5 mM 5′-F-IAA. LMW-PTP was immunoprecipitated and its redox state was evidenced by an anti-fluorescein immunoblot. An anti-LMW-PTP immunoblot was also performed for normalization. (B) LMW-PTP activity during ephrinA1 stimulation. Cells were treated as in A except that the activity of immunoprecipitated LMW-PTP was quantified by pNPP enzymatic assay. Half of each sample was used in an anti-LMW-PTP immunoblot for normalization and the normalized values obtained in three independent experiments are reported in the plot with SD. *p < 0.001 vs Fc. (C) Effect of genetic modulation of Rac activity on the redox state of LMW-PTP. Rac wt, Rac V12 and Rac N17 were overexpressed in PC3 cells. After 24 hours of starvation cells were detached, presuspended for 30 minutes in serum-free medium and kept in suspension or seeded onto FN-coated dishes for 45 minutes with or without ephrinA1-Fc. Cells were then treated as in (A) and an anti-fluorescein and anti-LMW-PTP were performed.
Figure 7
Role of redox signaling in 190RhoGAP phosphorylation during ephrinA1 stimulation. (A) After 24 hours of starvation PC3 cells were stimulated with 1 µg/ml ephrinA1-Fc for the indicated periods. Cells were then lysed in RIPA buffer and p190RhoGAP was immunoprecipitated. Anti-phosphotyrosine (4G10) and anti-p190RhoGAP immunoblots were performed. (B) Effect of LMW-PTP RNA interfering on p190RhoGAP phosphorylation: cells were treated as in A except for the presence of H2O2 1 µM (to promote the oxidation/inactivation of LMW-PTP). (C) Effect of H2O2 on p190RhoGAP phosphorylation: PC3 cells were transfected with LMW-PTP siRNA or scrambled siRNA and then treated as in (A). A western blot with anti-LMW-PTP specific antibodies was performed on cell lysates to check the silencing of the protein. D) Rho activity assay during ephrinA1/EphA2 signaling: PC3 cells, after 24 hours of starvation, were stimulated with ephrinA1-Fc in the presence or not of H2O2 1 µM. To create reducing conditions PC3 cells were pretreated with 20 mM NAC for 20 minutes, and then stimulated with ephrinA1-Fc. Cells were then lysed in RIPA buffer and RhoGTP was analyzed by a pull-down assay with Rhotekin-GST-agarose beads (B and D). A western blot with anti-RhoA specific antibodies was performed on cell lysates for normalization. The values of Rho activation obtained in four independent experiments are reported in the graph. The SD is indicated. *p < 0.001 vs Fc of the same treatment; #p < 0.005 vs Fc of the same treatment.
Figure 8
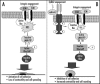
Proposed model of ephrinA1/EphA2 redox circuitry that controls integrin-dependent adhesion. (A) shows the molecular events that contribute to cell adhesion and spreading after integrin engagement; (B) shows the inhibitory effect of ephrinA1/EphA2 signaling leading to inhibition of integrin-dependent adhesion, increased contractility and cell rounding (see text for details).
Similar articles
- EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway.
Deroanne C, Vouret-Craviari V, Wang B, Pouysségur J. Deroanne C, et al. J Cell Sci. 2003 Apr 1;116(Pt 7):1367-76. doi: 10.1242/jcs.00308. J Cell Sci. 2003. PMID: 12615978 - Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion.
Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Chiarugi P, et al. J Cell Biol. 2003 Jun 9;161(5):933-44. doi: 10.1083/jcb.200211118. J Cell Biol. 2003. PMID: 12796479 Free PMC article. - EphrinA1 activates a Src/focal adhesion kinase-mediated motility response leading to rho-dependent actino/myosin contractility.
Parri M, Buricchi F, Giannoni E, Grimaldi G, Mello T, Raugei G, Ramponi G, Chiarugi P. Parri M, et al. J Biol Chem. 2007 Jul 6;282(27):19619-28. doi: 10.1074/jbc.M701319200. Epub 2007 Apr 22. J Biol Chem. 2007. PMID: 17449913 - The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting.
Wykosky J, Debinski W. Wykosky J, et al. Mol Cancer Res. 2008 Dec;6(12):1795-806. doi: 10.1158/1541-7786.MCR-08-0244. Mol Cancer Res. 2008. PMID: 19074825 Free PMC article. Review. - Reactive oxygen species as mediators of cell adhesion.
Chiarugi P. Chiarugi P. Ital J Biochem. 2003 Mar;52(1):28-32. Ital J Biochem. 2003. PMID: 12833635 Review.
Cited by
- Kinase-dependent and -independent roles of EphA2 in the regulation of prostate cancer invasion and metastasis.
Taddei ML, Parri M, Angelucci A, Onnis B, Bianchini F, Giannoni E, Raugei G, Calorini L, Rucci N, Teti A, Bologna M, Chiarugi P. Taddei ML, et al. Am J Pathol. 2009 Apr;174(4):1492-503. doi: 10.2353/ajpath.2009.080473. Epub 2009 Mar 5. Am J Pathol. 2009. PMID: 19264906 Free PMC article. - Exploring the potential of EphA2 receptor signaling pathway: a comprehensive review in cancer treatment.
Nehal M, Khatoon J, Akhtar S, Khan MKA. Nehal M, et al. Mol Biol Rep. 2024 Feb 23;51(1):337. doi: 10.1007/s11033-024-09298-8. Mol Biol Rep. 2024. PMID: 38393520 Review. - Self-Sustained Regulation or Self-Perpetuating Dysregulation: ROS-dependent HIF-YAP-Notch Signaling as a Double-Edged Sword on Stem Cell Physiology and Tumorigenesis.
Guo CL. Guo CL. Front Cell Dev Biol. 2022 Jun 14;10:862791. doi: 10.3389/fcell.2022.862791. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35774228 Free PMC article. Review. - Eph/ephrin signaling in cell-cell and cell-substrate adhesion.
Singh A, Winterbottom E, Daar IO. Singh A, et al. Front Biosci (Landmark Ed). 2012 Jan 1;17(2):473-97. doi: 10.2741/3939. Front Biosci (Landmark Ed). 2012. PMID: 22201756 Free PMC article. Review. - Activation of the low molecular weight protein tyrosine phosphatase in keratinocytes exposed to hyperosmotic stress.
Silva RA, Palladino MV, Cavalheiro RP, Machado D, Cruz BL, Paredes-Gamero EJ, Gomes-Marcondes MC, Zambuzzi WF, Vasques L, Nader HB, Souza AC, Justo GZ. Silva RA, et al. PLoS One. 2015 Mar 17;10(3):e0119020. doi: 10.1371/journal.pone.0119020. eCollection 2015. PLoS One. 2015. PMID: 25781955 Free PMC article.
References
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. - PubMed
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. - PubMed
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. - PubMed
- Schwartz MA, Ginsberg MH. Networks and crosstalk: Integrin signalling spreads. Nat Cell Biol. 2002;4:E65–E68. - PubMed
- Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: The oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161:933–944. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous