Glycerol monolaurate prevents mucosal SIV transmission - PubMed (original) (raw)
. 2009 Apr 23;458(7241):1034-8.
doi: 10.1038/nature07831. Epub 2009 Mar 4.
Jacob D Estes, Patrick M Schlievert, Lijie Duan, Amanda J Brosnahan, Peter J Southern, Cavan S Reilly, Marnie L Peterson, Nancy Schultz-Darken, Kevin G Brunner, Karla R Nephew, Stefan Pambuccian, Jeffrey D Lifson, John V Carlis, Ashley T Haase
Affiliations
- PMID: 19262509
- PMCID: PMC2785041
- DOI: 10.1038/nature07831
Glycerol monolaurate prevents mucosal SIV transmission
Qingsheng Li et al. Nature. 2009.
Abstract
Although there has been great progress in treating human immunodeficiency virus 1 (HIV-1) infection, preventing transmission has thus far proven an elusive goal. Indeed, recent trials of a candidate vaccine and microbicide have been disappointing, both for want of efficacy and concerns about increased rates of transmission. Nonetheless, studies of vaginal transmission in the simian immunodeficiency virus (SIV)-rhesus macaque (Macacca mulatta) model point to opportunities at the earliest stages of infection in which a vaccine or microbicide might be protective, by limiting the expansion of infected founder populations at the portal of entry. Here we show in this SIV-macaque model, that an outside-in endocervical mucosal signalling system, involving MIP-3alpha (also known as CCL20), plasmacytoid dendritic cells and CCR5(+ )cell-attracting chemokines produced by these cells, in combination with the innate immune and inflammatory responses to infection in both cervix and vagina, recruits CD4(+) T cells to fuel this obligate expansion. We then show that glycerol monolaurate-a widely used antimicrobial compound with inhibitory activity against the production of MIP-3alpha and other proinflammatory cytokines-can inhibit mucosal signalling and the innate and inflammatory response to HIV-1 and SIV in vitro, and in vivo it can protect rhesus macaques from acute infection despite repeated intra-vaginal exposure to high doses of SIV. This new approach, plausibly linked to interfering with innate host responses that recruit the target cells necessary to establish systemic infection, opens a promising new avenue for the development of effective interventions to block HIV-1 mucosal transmission.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Figures
Figure 1
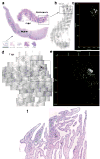
Mapping early expansion of infection in endocervix. SIV RNA+ cells appear black in transmitted light, green in reflected light and in maps. a–c. The arrow from thumbnail montage images (bottom of 1a) of cervix and vagina, 4–10dpi, points to an enlarged image and map of a single focus (box) of SIV RNA+ cells in endocervix, 4dpi. Counter clockwise-rotated image of focus (box) and map of X, Y coordinates (μm) of cell centroids to the right. d–e. Endocervical focus and map, 7dpi. f. Endocervical focus, 6dpi, SIV RNA+ cells (green) are concentrated in an inflammatory infiltrate (cells with dark staining nuclei).
Figure 2
Influx and infection of CD4+ T cells in cervix in early infection. a–c. Sections stained with anti-CD4. Note relative paucity of CD4+ cells in SIV-(negative animal) (a), or SIV inoculated animal 1 dpi (b), compared to increased numbers of CD4+ cells seen in infected animal at 4 dpi (c). d. SIV RNA+ cells in infiltrates are CD3+ T cells. Encircled SIV RNA+ cells (overlying black silver grains) are stained brown with anti-CD3.
Figure 3
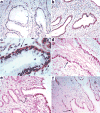
pDCs, cytokines and chemokines associated with endocervical epithelium following exposure to SIV. a. Uninfected animal. b–c. Rapid accumulation of pDCs beneath endocervical epithelium at 1 dpi shown at 10X (b) and 40X (c) original magnifications. pDCs stained brown with anti-CD123. Arrow in c points to the location of pDCs beneath the epithelium. d–e. Arrows point to subepithelial pDCs stained red with anti-interferon-α at 1 dpi (d) or anti-MIP-1β (e). f. Arrow points to MIP-3α+ endocervical epithelium (red) at 1 dpi.
Figure 4
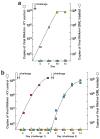
GML inhibits HIV-1 induced expression of MIP-3α and IL-8 in HVECs and in cervical vaginal fluids. a–b. R5 isolate of HIV-1 added to HVECs in the amounts indicated± GML. c–d. At the end of a six-month safety study, cervical vaginal fluids were collected with a swab that reproducibly adsorbed 0.1 ml of fluid from animals that received GML or K-Y warming gel in the am and pm on two successive days. MIP-3α and IL-8 measured by ELISA. Bars indicate standard errors of the mean.
Figure 5
GML prevents mucosal transmission and acute infection. a. Pilot experiment continuation of daily dosing safety study. Two animals treated with GML in K-Y warming gel (circles) and two treated with gel only (squares) were challenged twice (two arrows), 1-hour after treatment, with 105 TCID50 of SIV. Colors indicate individual animals. SIV RNA in plasma was measured to peak viremia, 14 dpi. b. Three animals treated with GML and three given K-Y warming gel were challenged as described in a. The animals that were not infected were treated and challenged again 4 weeks later, shown at the right.
Similar articles
- Glycerol Monolaurate Microbicide Protection against Repeat High-Dose SIV Vaginal Challenge.
Haase AT, Rakasz E, Schultz-Darken N, Nephew K, Weisgrau KL, Reilly CS, Li Q, Southern PJ, Rothenberger M, Peterson ML, Schlievert PM. Haase AT, et al. PLoS One. 2015 Jun 9;10(6):e0129465. doi: 10.1371/journal.pone.0129465. eCollection 2015. PLoS One. 2015. PMID: 26057743 Free PMC article. - Live simian immunodeficiency virus vaccine correlate of protection: immune complex-inhibitory Fc receptor interactions that reduce target cell availability.
Smith AJ, Wietgrefe SW, Shang L, Reilly CS, Southern PJ, Perkey KE, Duan L, Kohler H, Müller S, Robinson J, Carlis JV, Li Q, Johnson RP, Haase AT. Smith AJ, et al. J Immunol. 2014 Sep 15;193(6):3126-33. doi: 10.4049/jimmunol.1400822. Epub 2014 Aug 20. J Immunol. 2014. PMID: 25143442 Free PMC article. - Epithelium-innate immune cell axis in mucosal responses to SIV.
Shang L, Duan L, Perkey KE, Wietgrefe S, Zupancic M, Smith AJ, Southern PJ, Johnson RP, Haase AT. Shang L, et al. Mucosal Immunol. 2017 Mar;10(2):508-519. doi: 10.1038/mi.2016.62. Epub 2016 Jul 20. Mucosal Immunol. 2017. PMID: 27435105 Free PMC article. - Early events in sexual transmission of HIV and SIV and opportunities for interventions.
Haase AT. Haase AT. Annu Rev Med. 2011;62:127-39. doi: 10.1146/annurev-med-080709-124959. Annu Rev Med. 2011. PMID: 21054171 Review. - Localization of Simian immunodeficiency virus-infected cells in the genital tract of male and female Rhesus macaques.
Miller CJ. Miller CJ. J Reprod Immunol. 1998 Dec;41(1-2):331-9. doi: 10.1016/s0165-0378(98)00069-2. J Reprod Immunol. 1998. PMID: 10213321 Review.
Cited by
- Effect of hormonal contraception on the function of plasmacytoid dendritic cells and distribution of immune cell populations in the female reproductive tract.
Michel KG, Huijbregts RP, Gleason JL, Richter HE, Hel Z. Michel KG, et al. J Acquir Immune Defic Syndr. 2015 Apr 15;68(5):511-8. doi: 10.1097/QAI.0000000000000531. J Acquir Immune Defic Syndr. 2015. PMID: 25763784 Free PMC article. - Using nonhuman primates to model HIV transmission.
Fennessey CM, Keele BF. Fennessey CM, et al. Curr Opin HIV AIDS. 2013 Jul;8(4):280-7. doi: 10.1097/COH.0b013e328361cfff. Curr Opin HIV AIDS. 2013. PMID: 23666391 Free PMC article. Review. - The oral mucosa immune environment and oral transmission of HIV/SIV.
Wood LF, Chahroudi A, Chen HL, Jaspan HB, Sodora DL. Wood LF, et al. Immunol Rev. 2013 Jul;254(1):34-53. doi: 10.1111/imr.12078. Immunol Rev. 2013. PMID: 23772613 Free PMC article. Review. - Chemokine-mediated immune responses in the female genital tract mucosa.
Deruaz M, Luster AD. Deruaz M, et al. Immunol Cell Biol. 2015 Apr;93(4):347-54. doi: 10.1038/icb.2015.20. Epub 2015 Mar 17. Immunol Cell Biol. 2015. PMID: 25776842 Review. - HIV-1 imposes rigidity on blood and semen cytokine networks.
Lisco A, Introini A, Munawwar A, Vanpouille C, Grivel JC, Blank P, Singh S, Margolis L. Lisco A, et al. Am J Reprod Immunol. 2012 Dec;68(6):515-21. doi: 10.1111/aji.12015. Epub 2012 Sep 24. Am J Reprod Immunol. 2012. PMID: 23006048 Free PMC article.
References
- Fauci AS. 25 years of HIV. Nature. 2008;453:289–290. - PubMed
- Ledford H. HIV vaccine may raise risk. Nature. 2007;450:325. - PubMed
- Check E. Scientists rethink approach to HIV gels. Nature. 2007;446:12. - PubMed
- Cohen J. AIDS research. Microbicide fails to protect against HIV. Science. 2008;319:1026–1027. - PubMed
- Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI071976-02/AI/NIAID NIH HHS/United States
- RR020141-01/RR/NCRR NIH HHS/United States
- P51 RR000167-46S27592/RR/NCRR NIH HHS/United States
- P01 AI066314/AI/NIAID NIH HHS/United States
- P51 RR000167-440189/RR/NCRR NIH HHS/United States
- P01 AI066314-040003/AI/NIAID NIH HHS/United States
- R21 AI071976/AI/NIAID NIH HHS/United States
- P51 RR000167-440109/RR/NCRR NIH HHS/United States
- N01CO12400/CA/NCI NIH HHS/United States
- G20 RR022780/RR/NCRR NIH HHS/United States
- N01-CO-12400/CO/NCI NIH HHS/United States
- HHSN266200400088C/PHS HHS/United States
- G20 RR022780-01A1/RR/NCRR NIH HHS/United States
- C06 RR020141/RR/NCRR NIH HHS/United States
- C06 RR015459/RR/NCRR NIH HHS/United States
- P51 RR000167/RR/NCRR NIH HHS/United States
- RR15459-01/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials