Mepolizumab and exacerbations of refractory eosinophilic asthma - PubMed (original) (raw)
Randomized Controlled Trial
. 2009 Mar 5;360(10):973-84.
doi: 10.1056/NEJMoa0808991.
Affiliations
- PMID: 19264686
- PMCID: PMC3992367
- DOI: 10.1056/NEJMoa0808991
Randomized Controlled Trial
Mepolizumab and exacerbations of refractory eosinophilic asthma
Pranabashis Haldar et al. N Engl J Med. 2009.
Erratum in
- N Engl J Med. 2011 Feb 10;364(6):588
Abstract
Background: Exacerbations of asthma are associated with substantial morbidity and mortality and with considerable use of health care resources. Preventing exacerbations remains an important goal of therapy. There is evidence that eosinophilic inflammation of the airway is associated with the risk of exacerbations.
Methods: We conducted a randomized, double-blind, placebo-controlled, parallel-group study of 61 subjects who had refractory eosinophilic asthma and a history of recurrent severe exacerbations. Subjects received infusions of either mepolizumab, an anti-interleukin-5 monoclonal antibody (29 subjects), or placebo (32) at monthly intervals for 1 year. The primary outcome measure was the number of severe exacerbations per subject during the 50-week treatment phase. Secondary outcomes included a change in asthma symptoms, scores on the Asthma Quality of Life Questionnaire (AQLQ, in which scores range from 1 to 7, with lower values indicating more severe impairment and a change of 0.5 unit considered to be clinically important), forced expiratory volume in 1 second (FEV(1)) after use of a bronchodilator, airway hyperresponsiveness, and eosinophil counts in the blood and sputum.
Results: Mepolizumab was associated with significantly fewer severe exacerbations than placebo over the course of 50 weeks (2.0 vs. 3.4 mean exacerbations per subject; relative risk, 0.57; 95% confidence interval [CI], 0.32 to 0.92; P=0.02) and with a significant improvement in the score on the AQLQ (mean increase from baseline, 0.55 vs. 0.19; mean difference between groups, 0.35; 95% CI, 0.08 to 0.62; P=0.02). Mepolizumab significantly lowered eosinophil counts in the blood (P<0.001) and sputum (P=0.002). There were no significant differences between the groups with respect to symptoms, FEV(1) after bronchodilator use, or airway hyperresponsiveness. The only serious adverse events reported were hospitalizations for acute severe asthma.
Conclusions: Mepolizumab therapy reduces exacerbations and improves AQLQ scores in patients with refractory eosinophilic asthma. The results of our study suggest that eosinophils have a role as important effector cells in the pathogenesis of severe exacerbations of asthma in this patient population. (Current Controlled Trials number, ISRCTN75169762.)
2009 Massachusetts Medical Society
Figures
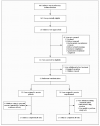
Figure 1. Numbers of Patients Who Were Screened, Enrolled, and Assigned to a Study Group and Who Completed the Study
All subjects were recruited from a database of patients who were attending our refractory-asthma clinic. Of the 449 persons in the database, 52.3% had a history of sputum eosinophilia of more than 3% on at least one occasion in the previous 2 years, and 63.4% of these patients had been treated with two or more courses of oral corticosteroid therapy in the previous 12 months.
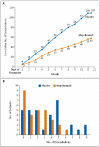
Figure 2. Severe Exacerbations during the Course of the Study
Panel A shows the cumulative number of severe exacerbations that occurred in each study group over the course of 50 weeks. Panel B shows the distribution of the number of exacerbations among subjects in each study group during the treatment period of the study. The mean number of exacerbations per subject over the course of the 50-week treatment period was 2.0 in the mepolizumab group, as compared with 3.4 in the placebo group (relative risk, 0.57; 95% confidence interval, 0.32 to 0.92; P=0.02).
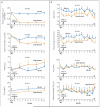
Figure 3. Comparison of Secondary Outcomes between Study Groups
Panel A shows geometric mean (log10 SE) eosinophil counts in blood and sputum specimens, the provocative concentration of methacholine required to cause a 20% fall in FEV1 (PC20), and mean scores on the Asthma Quality of Life Questionnaire (AQLQ). The AQLQ comprises 32 items, each of which is scored on a scale of 1 to 7, with higher scores indicating better asthma-related quality of life. The items are grouped into four domains, and the reported score is the mean of responses across the four domains. Longitudinal data on PC20 were available for 16 and 18 subjects in the mepolizumab and placebo groups, respectively. Panel B shows mean symptom scores, the forced expiratory volume in 1 second (FEV1) after bronchodilator use, and the geometric mean (log10 SE) fraction of exhaled nitric oxide at an expiratory flow of 50 ml per second (F
e
NO) before, during, and after the treatment phase of the study. The score on the modified Juniper Asthma Control Questionnaire (JACQ) represents the mean of responses to five questions about daytime and nighttime symptoms and limitation of activities, with each response scored on a scale of 0 to 6; higher scores represent worse symptoms. The mean visual-analogue score represents the total scores divided by 3 for cough, wheezing, and breathlessness, each of which was assessed on a 100-mm scale, with “no symptoms” at one end and “the worst symptoms ever” at the other end. Higher scores indicate worse symptoms. These factors were evaluated before and after administration of 0.5 mg of prednisolone per kilogram per day, with a maximum dose of 40 mg per day, for 14 days, at the beginning and end of the treatment phase. The purple bars represent the 2-week course of prednisolone therapy. P values are for the mean difference between the groups for the change from baseline to the mean or geometric mean of the post-treatment values. Further details are given in Tables 1 and 2 in the Supplementary Appendix. The term ppb denotes parts per billion.
Comment in
- Eosinophils in asthma--closing the loop or opening the door?
Wenzel SE. Wenzel SE. N Engl J Med. 2009 Mar 5;360(10):1026-8. doi: 10.1056/NEJMe0900334. N Engl J Med. 2009. PMID: 19264692 No abstract available. - Mepolizumab for difficult-to-control asthma with persistent sputum eosinophilia.
Antoniu SA. Antoniu SA. Expert Opin Investig Drugs. 2009 Jun;18(6):869-71. doi: 10.1517/13543780902922678. Expert Opin Investig Drugs. 2009. PMID: 19426126 Clinical Trial. - Anti-interleukin-5 therapy and severe asthma.
Zarogiannis S, Gourgoulianis KI, Kostikas K. Zarogiannis S, et al. N Engl J Med. 2009 Jun 11;360(24):2576; author reply 2577. doi: 10.1056/NEJMc090685. N Engl J Med. 2009. PMID: 19516040 No abstract available. - Anti-interleukin-5 therapy and severe asthma.
Szczeklik A. Szczeklik A. N Engl J Med. 2009 Jun 11;360(24):2576-7; author reply 2577. N Engl J Med. 2009. PMID: 19522046 No abstract available. - Anti-interleukin-5 therapy and severe asthma.
Gleich GJ. Gleich GJ. N Engl J Med. 2009 Jun 11;360(24):2577; author reply 2578. N Engl J Med. 2009. PMID: 19522047 No abstract available.
Similar articles
- Mepolizumab for prednisone-dependent asthma with sputum eosinophilia.
Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Nair P, et al. N Engl J Med. 2009 Mar 5;360(10):985-93. doi: 10.1056/NEJMoa0805435. N Engl J Med. 2009. PMID: 19264687 Clinical Trial. - Mepolizumab treatment in patients with severe eosinophilic asthma.
Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, Chanez P; MENSA Investigators. Ortega HG, et al. N Engl J Med. 2014 Sep 25;371(13):1198-207. doi: 10.1056/NEJMoa1403290. Epub 2014 Sep 8. N Engl J Med. 2014. PMID: 25199059 Clinical Trial. - Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial.
Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Pavord ID, et al. Lancet. 2012 Aug 18;380(9842):651-9. doi: 10.1016/S0140-6736(12)60988-X. Lancet. 2012. PMID: 22901886 Clinical Trial. - Mepolizumab: 240563, anti-IL-5 monoclonal antibody - GlaxoSmithKline, anti-interleukin-5 monoclonal antibody - GlaxoSmithKline, SB 240563.
[No authors listed] [No authors listed] Drugs R D. 2008;9(2):125-30. doi: 10.2165/00126839-200809020-00006. Drugs R D. 2008. PMID: 18298130 Review. - Mepolizumab versus placebo for asthma.
Powell C, Milan SJ, Dwan K, Bax L, Walters N. Powell C, et al. Cochrane Database Syst Rev. 2015 Jul 27;(7):CD010834. doi: 10.1002/14651858.CD010834.pub2. Cochrane Database Syst Rev. 2015. PMID: 26214266 Updated. Review.
Cited by
- Controlled human exposures: a review and comparison of the health effects of diesel exhaust and wood smoke.
Long E, Rider CF, Carlsten C. Long E, et al. Part Fibre Toxicol. 2024 Oct 23;21(1):44. doi: 10.1186/s12989-024-00603-8. Part Fibre Toxicol. 2024. PMID: 39444041 Free PMC article. Review. - Anti-aminoacyl-tRNA synthetase-interacting multifunctional protein-1 antibody improves airway inflammation in mice with house dust mite induced asthma.
Kim SR, Um YJ, Chung SI, Jeong KY, Park HJ, Park KH, Park JW, Park SG, Lee JH. Kim SR, et al. World Allergy Organ J. 2024 Aug 22;17(9):100956. doi: 10.1016/j.waojou.2024.100956. eCollection 2024 Sep. World Allergy Organ J. 2024. PMID: 39262899 Free PMC article. - Mepolizumab in Severe Pediatric Asthma: Certainties and Doubts through a Single-Center Experience and Review of the Literature.
Maglione M, Borrelli M, Dorato A, Cimbalo C, Del Giudice LA, Santamaria F. Maglione M, et al. Children (Basel). 2024 Jul 25;11(8):895. doi: 10.3390/children11080895. Children (Basel). 2024. PMID: 39201830 Free PMC article. - SingleNucleotide Polymorphisms as Biomarkers of Mepolizumab and Benralizumab Treatment Response in Severe Eosinophilic Asthma.
Rojo-Tolosa S, Sánchez-Martínez JA, Caballero-Vázquez A, Pineda-Lancheros LE, González-Gutiérrez MV, Pérez-Ramírez C, Jiménez-Morales A, Morales-García C. Rojo-Tolosa S, et al. Int J Mol Sci. 2024 Jul 26;25(15):8139. doi: 10.3390/ijms25158139. Int J Mol Sci. 2024. PMID: 39125709 Free PMC article. - Genomic Profiling for Predictive Treatment Strategies in Fibrotic Interstitial Lung Disease.
Perrotta F, Sanduzzi Zamparelli S, D'Agnano V, Montella A, Fomez R, Pagliaro R, Schiattarella A, Cazzola M, Bianco A, Mariniello DF. Perrotta F, et al. Biomedicines. 2024 Jun 21;12(7):1384. doi: 10.3390/biomedicines12071384. Biomedicines. 2024. PMID: 39061958 Free PMC article. Review.
References
- Global Initiative for Asthma (GINA) [Accessed February 9, 2009];Global strategy for asthma management and prevention. 2007 http://www.ginasthma.org
- Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med. 2000;161:64–72. - PubMed
- Deykin A, Lazarus SC, Fahy JV, et al. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol. 2005;115:720–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical