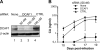The human immunodeficiency virus type 2 Vpx protein usurps the CUL4A-DDB1 DCAF1 ubiquitin ligase to overcome a postentry block in macrophage infection - PubMed (original) (raw)
. 2009 May;83(10):4854-60.
doi: 10.1128/JVI.00187-09. Epub 2009 Mar 4.
Diana Ayinde, Annie David, Erwann Le Rouzic, Marina Morel, Gilles Collin, Diane Descamps, Florence Damond, Françoise Brun-Vezinet, Sebastien Nisole, Florence Margottin-Goguet, Gianfranco Pancino, Catherine Transy
Affiliations
- PMID: 19264781
- PMCID: PMC2682070
- DOI: 10.1128/JVI.00187-09
The human immunodeficiency virus type 2 Vpx protein usurps the CUL4A-DDB1 DCAF1 ubiquitin ligase to overcome a postentry block in macrophage infection
Anna Bergamaschi et al. J Virol. 2009 May.
Abstract
The human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) genomes encode several auxiliary proteins that have increasingly shown their importance in the virus-host relationship. One of these proteins, Vpx, is unique to the HIV-2/SIVsm lineage and is critical for viral replication in macrophages. The functional basis for this requirement, as well as the Vpx mode of action, has remained unexplained, and it is all the more enigmatic that HIV type 1 (HIV-1), which has no Vpx counterpart, can infect macrophages. Here, we underscore DCAF1 as a critical host effector of Vpx in its ability to mediate infection and long-term replication of HIV-2 in human macrophages. Vpx assembles with the CUL4A-DDB1 ubiquitin ligase through DCAF1 recruitment. Precluding Vpx present in the incoming virions from recruiting DCAF1 in target macrophages leads to a postentry block characterized by defective accumulation of HIV-2 reverse transcripts. In addition, Vpx from SIVsm functionally complements Vpx-defective HIV-2 in a DCAF1-binding-dependent manner. Altogether, our data point to a mechanism in which Vpx diverts the Cul4A-DDB1(DCAF1) ligase to inactivate an evolutionarily conserved factor, which restricts macrophage infection by HIV-2 and closely related simian viruses.
Figures
FIG. 1.
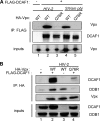
The HIV-2 Vpx protein recruits DDB1 through DCAF1 binding in a Q76 residue-dependent manner. Cell lysates were prepared from HeLa cells expressing the indicated HA-tagged Vpx proteins along with FLAG-tagged DCAF1, where indicated. Crude cell lysates (inputs) and immunoprecipitates (IP) were analyzed by WB to reveal the transfected tagged proteins (A and B) and endogenous DDB1 (B).
FIG. 2.
The Q76R mutation in Vpx strongly impairs HIV-2 replication in human primary macrophages. (A) The Q76R mutation impairs HIV-2 replication in macrophages to an extent similar to that with the Vpx deletion. Macrophages (left) or CD4 T cells (right) were infected with the indicated HIV-2 strains, and the CA contents of the culture supernatants were monitored over time by ELISA. Shown is a representative experiment out of four performed with macrophages from different donors. The results are expressed as means of the CA levels from triplicate samples ± standard deviations. (B) The Q76R Vpx mutant is efficiently encapsidated into virions. The culture supernatants of 293T cells were harvested 48 h after transfection with the indicated HIV-2 proviral plasmids and were ultracentrifuged through a 20% sucrose cushion. After lysis in sodium dodecyl sulfate, the pelleted virions were analyzed by WB using anti-Vpx and anti-CA antibodies. Δ, lack of Vpx.
FIG. 3.
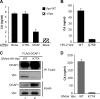
DCAF1 knockdown inhibits HIV-2 replication in macrophages. Macrophages were mock treated or transfected with either DCAF1-specific or control (CTRL) siRNA at the indicated concentrations. (A) DCAF1 was detected by immunoblot analysis of cell lysates prepared 72 h posttransfection. (B) Macrophages were transfected with the indicated siRNAs 72 h prior to infection with WT HIV-2, and viral CA in culture supernatants was monitored over time as described in the legend to Fig. 2.
FIG. 4.
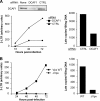
The recruitment of DCAF1 by incoming Vpx is critical for efficient macrophage transduction by HIV-2. (A) VSV-G-pseudotyped HIV-2 strains were produced by cotransfection of 293T cells with Env-defective proviral DNA and a plasmid encoding the VSV-G envelope. Macrophages were mock treated or transfected with either DCAF1-specific or control (CTRL) siRNA 72 h prior to transduction with the indicated HIV-2 particles. The intracellular CA content was measured by ELISA 72 h postinfection. Shown is a representative experiment out of four performed with macrophages from different donors. The bars represent mean values calculated from triplicate cell lysates, and the corresponding standard deviations are indicated. (B) The VSV-G-pseudotyped HIV-2 particles were produced as for panel A using a Vpx-defective proviral DNA, along with a construct expressing the indicated HIV-2 Vpx proteins. The content in intracellular CA was measured 96 h posttransduction. (C) Vpx WT and Vpx K77A from SIVsmPBj were expressed as HA-tagged proteins and compared for their abilities to interact with FLAG-DCAF1 using coimmunoprecipitation as described in the legend to Fig. 1B. (D) Macrophages were transduced with VSV-G-pseudotyped HIV-2 particles complemented with the indicated SIVsmPbj Vpx proteins, and the content in intracellular CA was measured as for panel B. Similar results were obtained using macrophages from four different donors.
FIG. 5.
Both Vpx and DCAF1 are required in the preintegration steps of the HIV-2 life cycle in macrophages. (A) Decreased amounts of two-LTR circles and late RT products in DCAF1-depleted macrophages. Macrophages were transfected with DCAF1-specific or control (CTRL) siRNAs 72 h prior to infection with VSV-G-pseudotyped HIV-2 particles. The accumulation of two-LTR circles was monitored over time using real-time PCR (left). The products were quantified by comparison to a standard curve generated by serial dilutions of DNA from HIV-2-infected HeLa P4P cells, and the results are expressed as arbitrary units. Levels of late RT products were assessed by real-time PCR 72 h postinfection, when accumulation was maximal (right). All PCR experiments were performed on duplicate samples of cell DNA. (B) Defective HIV-2 RT in the absence of Vpx. Macrophages were infected with the indicated VSV-G-pseudotyped HIV-2 virions. DNA was extracted and analyzed as described for panel A. Similar results were obtained using macrophages from four different donors.
FIG. 6.
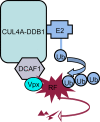
Presumed mode of action of HIV-2 Vpx in macrophage infection. Vpx connects the DCAF1 adaptor of the CUL4A-DDB1 ubiquitin (Ub) ligase to a macrophage-specific restriction factor (RF), which targets the HIV-2/SIVsm lentiviruses. As a result, RF is polyubiquitinated and is subsequently degraded by the proteasome.
Similar articles
- Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection.
Srivastava S, Swanson SK, Manel N, Florens L, Washburn MP, Skowronski J. Srivastava S, et al. PLoS Pathog. 2008 May 9;4(5):e1000059. doi: 10.1371/journal.ppat.1000059. PLoS Pathog. 2008. PMID: 18464893 Free PMC article. - Inhibition of Vpx-Mediated SAMHD1 and Vpr-Mediated Host Helicase Transcription Factor Degradation by Selective Disruption of Viral CRL4 (DCAF1) E3 Ubiquitin Ligase Assembly.
Wang H, Guo H, Su J, Rui Y, Zheng W, Gao W, Zhang W, Li Z, Liu G, Markham RB, Wei W, Yu XF. Wang H, et al. J Virol. 2017 Apr 13;91(9):e00225-17. doi: 10.1128/JVI.00225-17. Print 2017 May 1. J Virol. 2017. PMID: 28202763 Free PMC article. - Cullin4A and cullin4B are interchangeable for HIV Vpr and Vpx action through the CRL4 ubiquitin ligase complex.
Sharifi HJ, Furuya AK, Jellinger RM, Nekorchuk MD, de Noronha CM. Sharifi HJ, et al. J Virol. 2014 Jun;88(12):6944-58. doi: 10.1128/JVI.00241-14. Epub 2014 Apr 9. J Virol. 2014. PMID: 24719410 Free PMC article. - Lentivirus Vpr and Vpx accessory proteins usurp the cullin4-DDB1 (DCAF1) E3 ubiquitin ligase.
Romani B, Cohen EA. Romani B, et al. Curr Opin Virol. 2012 Dec;2(6):755-63. doi: 10.1016/j.coviro.2012.09.010. Epub 2012 Oct 10. Curr Opin Virol. 2012. PMID: 23062609 Free PMC article. Review. - The functions of the HIV1 protein Vpr and its action through the DCAF1.DDB1.Cullin4 ubiquitin ligase.
Casey L, Wen X, de Noronha CM. Casey L, et al. Cytokine. 2010 Jul;51(1):1-9. doi: 10.1016/j.cyto.2010.02.018. Epub 2010 Mar 27. Cytokine. 2010. PMID: 20347598 Free PMC article. Review.
Cited by
- Formation of mobile chromatin-associated nuclear foci containing HIV-1 Vpr and VPRBP is critical for the induction of G2 cell cycle arrest.
Belzile JP, Abrahamyan LG, Gérard FC, Rougeau N, Cohen EA. Belzile JP, et al. PLoS Pathog. 2010 Sep 2;6(9):e1001080. doi: 10.1371/journal.ppat.1001080. PLoS Pathog. 2010. PMID: 20824083 Free PMC article. - Molecular insight into how HIV-1 Vpr protein impairs cell growth through two genetically distinct pathways.
Maudet C, Bertrand M, Le Rouzic E, Lahouassa H, Ayinde D, Nisole S, Goujon C, Cimarelli A, Margottin-Goguet F, Transy C. Maudet C, et al. J Biol Chem. 2011 Jul 8;286(27):23742-52. doi: 10.1074/jbc.M111.220780. Epub 2011 May 12. J Biol Chem. 2011. PMID: 21566118 Free PMC article. - The Fourth Major Restriction Factor Against HIV/SIV.
Nomaguchi M, Fujita M, Adachi A. Nomaguchi M, et al. Front Microbiol. 2011 Jun 10;2:132. doi: 10.3389/fmicb.2011.00132. eCollection 2011. Front Microbiol. 2011. PMID: 21713064 Free PMC article. No abstract available. - Vpx overcomes a SAMHD1-independent block to HIV reverse transcription that is specific to resting CD4 T cells.
Baldauf HM, Stegmann L, Schwarz SM, Ambiel I, Trotard M, Martin M, Burggraf M, Lenzi GM, Lejk H, Pan X, Fregoso OI, Lim ES, Abraham L, Nguyen LA, Rutsch F, König R, Kim B, Emerman M, Fackler OT, Keppler OT. Baldauf HM, et al. Proc Natl Acad Sci U S A. 2017 Mar 7;114(10):2729-2734. doi: 10.1073/pnas.1613635114. Epub 2017 Feb 22. Proc Natl Acad Sci U S A. 2017. PMID: 28228523 Free PMC article. - Positive selection in dNTPase SAMHD1 throughout mammalian evolution.
Monit C, Morris ER, Ruis C, Szafran B, Thiltgen G, Tsai MC, Mitchison NA, Bishop KN, Stoye JP, Taylor IA, Fassati A, Goldstein RA. Monit C, et al. Proc Natl Acad Sci U S A. 2019 Sep 10;116(37):18647-18654. doi: 10.1073/pnas.1908755116. Epub 2019 Aug 26. Proc Natl Acad Sci U S A. 2019. PMID: 31451672 Free PMC article.
References
- Balliet, J. W., D. L. Kolson, G. Eiger, F. M. Kim, K. A. McGann, A. Srinivasan, and R. Collman. 1994. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology 200623-631. - PubMed
- Brass, A. L., D. M. Dykxhoorn, Y. Benita, N. Yan, A. Engelman, R. J. Xavier, J. Lieberman, and S. J. Elledge. 2008. Identification of host proteins required for HIV infection through a functional genomic screen. Science 319921-926. - PubMed
- Clavel, F., D. Guetard, F. Brun-Vezinet, S. Chamaret, M. A. Rey, M. O. Santos-Ferreira, A. G. Laurent, C. Dauguet, C. Katlama, C. Rouzioux, et al. 1986. Isolation of a new human retrovirus from West African patients with AIDS. Science 233343-346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases