CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions - PubMed (original) (raw)
CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions
Kyle J Eash et al. Blood. 2009.
Abstract
The number of neutrophils in the blood is tightly regulated to ensure adequate protection against microbial pathogens while minimizing damage to host tissue. Neutrophil homeostasis in the blood is achieved through a balance of neutrophil production, release from the bone marrow, and clearance from the circulation. Accumulating evidence suggests that signaling by CXCL12, through its major receptor CXCR4, plays a key role in maintaining neutrophil homeostasis. Herein, we generated mice with a myeloid lineage-restricted deletion of CXCR4 to define the mechanisms by which CXCR4 signals regulate this process. We show that CXCR4 negatively regulates neutrophil release from the bone marrow in a cell-autonomous fashion. However, CXCR4 is dispensable for neutrophil clearance from the circulation. Neutrophil mobilization responses to granulocyte colony-stimulating factor (G-CSF), CXCL2, or Listeria monocytogenes infection are absent or impaired, suggesting that disruption of CXCR4 signaling may be a common step mediating neutrophil release. Collectively, these data suggest that CXCR4 signaling maintains neutrophil homeostasis in the blood under both basal and stress granulopoiesis conditions primarily by regulating neutrophil release from the bone marrow.
Figures
Figure 1
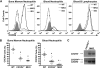
CXCR4 is efficiently deleted in neutrophils from LysMCre/+ CXCR4flox/− (MKO) mice. (A) Representative histograms showing cell-surface CXCR4 expression in the mature neutrophil (Gr-1brightSSChi) population from bone marrow or peripheral blood or the peripheral blood B-lymphocyte (B-220+) population in wild-type (WT) or MKO mice. The isotype control (ISO) is shown in gray. (B) Cell-surface CXCR4 expression in the mature neutrophil population in the bone marrow or peripheral blood. Data represent the mean ± SEM. #P < .05 compared with wild-type mice; *P < .05 compared with CXCR4+/− mice. (C) Genomic DNA was isolated from MKO or control (CXCR4 flox/− without LysMCre) blood neutrophils and the CXCR4 gene amplified using primers that specifically detected the deleted (CXCR4_Δ), floxed (CXCR4flox), or null (CXCR4_−) CXCR4 alleles.
Figure 2
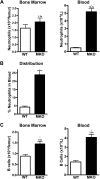
Basal granulopoiesis in MKO mice is characterized by a shift of neutrophils from the bone marrow to the blood. (A,B) The number of mature neutrophils (Gr-1brightSSChi) in the blood and bone marrow was quantified by flow cytometry in mice of the indicated genotype. (C) The neutrophil distribution index (NDI) was calculated to estimate the percentage of total body neutrophils in the blood, using the following formula: NDI = blood neutrophils/(blood + bone marrow neutrophils). Data represent the mean ± SEM. #P < .05 compared with all other groups.
Figure 3
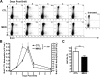
Blood neutrophil half-life in MKO mice is normal. BrdU (2 mg) was administered to control (CTL) or MKO mice by a single intraperitoneal injection. Peripheral blood was obtained at the indicated time points and the number of BrdU+ Gr-1bright cells determined by flow cytometry. (A) Representative dot plots showing BrdU staining in the Gr-1bright (mature neutrophil) population. The numbers shown indicate the percentage of Gr-1bright cells that were BrdU+. (B) The absolute number of BrdU+ Gr-1bright neutrophils in the blood is shown. (C) Neutrophil half-life (t1/2) in the blood was calculated according to the formulas t1/2 = ln 2/λ and nt = n0e−λt where n0 is the number at a given time, nt is the number t hours later, and λ is the decay constant. The data shown represent the mean ± SEM of n = 10 to 11 mice in each group. *P < .05 compared with control mice at the same time point. ns indicates not significant.
Figure 4
MKO neutrophils have impaired homing to the bone marrow. Bone marrow cells (8-10 × 106) from control (CTL) or MKO mice carrying the Ly 5.2 allele were adoptively transferred to WT recipients carrying the Ly 5.1 allele, enabling detection of infused neutrophils in the bone marrow by flow cytometry using an allele-specific CD45.2 antibody. (A) Representative dot plots showing donor neutrophils as the percentage of total bone marrow neutrophils 1.5 to 2.5 hours after infusion. (B) The percentage of transferred control or MKO neutrophils present in the bone marrow of recipient mice. Data represent the mean ± SEM of n = 8 to 12 recipients for each genotype from 3 separate experiments. *P < .05 compared with control neutrophils.
Figure 5
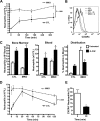
Trafficking of MKO neutrophils is altered in mixed chimeras. Whole bone marrow from wild-type (WT, Ly5.1) and MKO (Ly5.2) mice was mixed in a 1:1 ratio and transplanted into lethally irradiated wild-type (Ly5.1) recipients. After hematopoietic reconstitution (8-10 weeks), the bone marrow and blood were analyzed by flow cytometry. The numbers at the top of the columns indicate the fold increase over the wild-type cells. (A) Mature neutrophils (Gr-1bright CD115−). (B) Neutrophil distribution index. (C) B lymphocytes (B220+). The data represent the mean ± SEM of n = 18 recipients from 2 separate transplants. *P < .05 compared with wild-type cells.
Figure 6
Neutrophil mobilization by G-CSF or GROβ is abrogated in MKO mice. (A) Mice (n = 5 per group) were given a single subcutaneous injection of G-CSF (125 μg/kg) and the absolute neutrophil count measured at the indicated times. †P < .05 compared with time 0. (B) Representative histograms showing cell surface CXCR4 expression on blood neutrophils from control (CTL) or MKO mice at baseline and 65 minutes after a single dose of G-CSF (+G). (C) Mice (n = 8-11 per group) were treated with G-CSF (125 μg/kg per day, twice daily injections) for 5 days, and neutrophils in the bone marrow and blood were quantified. The calculated neutrophil distribution index is shown in the far right panel. *P < .05 compared with control mice at the same time point; #P < .05 compared with untreated mice of the same genotype. (D) Mice (n = 9-12 per group) were given a single subcutaneous injection of GROβ (100 μg/kg), and the absolute neutrophil count was determined at the indicated times. †P < .05 compared with time 0. (E) CXCR4 cell-surface expression on peripheral blood neutrophils from control mice was determined by flow cytometry at baseline and at the time of peak mobilization, 30 minutes after GROβ administration. **P < .001. Data represent the mean ± SEM.
Figure 7
MKO mice have impaired blood neutrophil mobilization but normal neutrophil recruitment to the peritoneum in response to Listeria infection. Control (CTL) or MKO mice were infected intraperitoneally with L monocytogenes. (A) Survival was assessed in mice (n = 12 per group) from 2 separate infections with 9.8 to 11.2 × 105 CFU of bacteria. (B) The bacterial titer in the spleen and liver of control and MKO mice (n = 8 per group) was determined 72 hours after infection with 2.1 to 7.2 × 105 CFU of bacteria. (C) Blood neutrophil counts were assessed by flow cytometry at the indicated times after infection with 2.1 to 9.8 × 105 CFU of bacteria (n = 8-19 mice per group depending on the time). (D) Shown is the number of neutrophils in the peritoneum at the indicated times after infection with 2.1 to 7.2 × 105 CFU of bacteria (n = 5-8 mice per group depending on the time). Data represent the mean ± SEM. †P < .05 compared with time 0.
Similar articles
- CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow.
Eash KJ, Greenbaum AM, Gopalan PK, Link DC. Eash KJ, et al. J Clin Invest. 2010 Jul;120(7):2423-31. doi: 10.1172/JCI41649. J Clin Invest. 2010. PMID: 20516641 Free PMC article. - Regulation of neutrophil trafficking from the bone marrow.
Day RB, Link DC. Day RB, et al. Cell Mol Life Sci. 2012 May;69(9):1415-23. doi: 10.1007/s00018-011-0870-8. Epub 2011 Nov 2. Cell Mol Life Sci. 2012. PMID: 22045556 Free PMC article. Review. - GRK6 deficiency is associated with enhanced CXCR4-mediated neutrophil chemotaxis in vitro and impaired responsiveness to G-CSF in vivo.
Vroon A, Heijnen CJ, Raatgever R, Touw IP, Ploemacher RE, Premont RT, Kavelaars A. Vroon A, et al. J Leukoc Biol. 2004 Apr;75(4):698-704. doi: 10.1189/jlb.0703320. Epub 2004 Jan 2. J Leukoc Biol. 2004. PMID: 14704365 - CXCR4, the master regulator of neutrophil trafficking in homeostasis and disease.
De Filippo K, Rankin SM. De Filippo K, et al. Eur J Clin Invest. 2018 Nov;48 Suppl 2(Suppl Suppl 2):e12949. doi: 10.1111/eci.12949. Epub 2018 May 23. Eur J Clin Invest. 2018. PMID: 29734477 Free PMC article. Review. - Regulation of neutrophil homeostasis.
Christopher MJ, Link DC. Christopher MJ, et al. Curr Opin Hematol. 2007 Jan;14(1):3-8. doi: 10.1097/00062752-200701000-00003. Curr Opin Hematol. 2007. PMID: 17133093 Review.
Cited by
- Chemokines as Regulators of Neutrophils: Focus on Tumors, Therapeutic Targeting, and Immunotherapy.
Bonecchi R, Mantovani A, Jaillon S. Bonecchi R, et al. Cancers (Basel). 2022 Jan 28;14(3):680. doi: 10.3390/cancers14030680. Cancers (Basel). 2022. PMID: 35158948 Free PMC article. Review. - The Effects of 16 Weeks of Exercise Training on Neutrophil Functions in Breast Cancer Survivors.
Bartlett DB, Hanson ED, Lee JT, Wagoner CW, Harrell EP, Sullivan SA, Bates LC, Alzer MS, Amatuli DJ, Deal AM, Jensen BC, MacDonald G, Deal MA, Muss HB, Nyrop KA, Battaglini CL. Bartlett DB, et al. Front Immunol. 2021 Oct 27;12:733101. doi: 10.3389/fimmu.2021.733101. eCollection 2021. Front Immunol. 2021. PMID: 34777343 Free PMC article. - CXCL1 regulates neutrophil homeostasis in pneumonia-derived sepsis caused by Streptococcus pneumoniae serotype 3.
Paudel S, Baral P, Ghimire L, Bergeron S, Jin L, DeCorte JA, Le JT, Cai S, Jeyaseelan S. Paudel S, et al. Blood. 2019 Mar 21;133(12):1335-1345. doi: 10.1182/blood-2018-10-878082. Epub 2019 Feb 5. Blood. 2019. PMID: 30723078 Free PMC article. - Concepts of GPCR-controlled navigation in the immune system.
Lämmermann T, Kastenmüller W. Lämmermann T, et al. Immunol Rev. 2019 May;289(1):205-231. doi: 10.1111/imr.12752. Immunol Rev. 2019. PMID: 30977203 Free PMC article. Review. - Bcl3 prevents acute inflammatory lung injury in mice by restraining emergency granulopoiesis.
Kreisel D, Sugimoto S, Tietjens J, Zhu J, Yamamoto S, Krupnick AS, Carmody RJ, Gelman AE. Kreisel D, et al. J Clin Invest. 2011 Jan;121(1):265-76. doi: 10.1172/JCI42596. Epub 2010 Dec 13. J Clin Invest. 2011. PMID: 21157041 Free PMC article.
References
- Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14:3–8. - PubMed
- Lord B, Molineux G, Pojda Z, Souza L, Mermod J, Dexter T. Myeloid cell kinetics in mice treated with recombinant interleukin-3, granulocyte colony-stimulating factor (CSF), or granulocyte-macrophage CSF in vivo. Blood. 1991;77:2154–2159. - PubMed
- Lord BI. Myeloid cell kinetics in response to haemopoietic growth factors. Baillieres Clin Haematol. 1992;5:533–550. - PubMed
- Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–861. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 AI079011/AI/NIAID NIH HHS/United States
- R01 HL060772/HL/NHLBI NIH HHS/United States
- K08AI079011-01/AI/NIAID NIH HHS/United States
- R01 HL60772/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases