Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice - PubMed (original) (raw)
Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice
Danielle A Simmons et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Cognitive problems occur in asymptomatic gene carriers of Huntington's disease (HD), and mouse models of the disease exhibit impaired learning and substantial deficits in the cytoskeletal changes that stabilize long-term potentiation (LTP). The latter effects may be related to the decreased production of brain-derived neurotrophic factor (BDNF) associated with the HD mutation. This study asked whether up-regulating endogenous BDNF levels with an ampakine, a positive modulator of AMPA-type glutamate receptors, rescues plasticity and reduces learning problems in HD (CAG140) mice. Twice-daily injections of a short half-life ampakine normalized BDNF levels, activity-driven actin polymerization in dendritic spines, and LTP stabilization in 8-week-old mutants. Comparable results were obtained in 16-week-old HD mice with more severe LTP deficits. Ampakine treatments had no measurable effect on the decreased locomotor activity observed in the mutants but offset their impairments in long-term memory. Given that ampakines are well tolerated in clinical trials and were effective in this study after brief exposures, these results suggest a novel strategy for chronic treatment of the cognitive difficulties that occur in the early stages of HD.
Conflict of interest statement
Conflict of interest statement: G.L. is a consultant for and has financial interests in Cortex Pharmaceuticals, Inc., which provided the ampakine used in this study.
Figures
Fig. 1.
Exogenous BDNF reverses LTP deficits in CAG140 mice. The initial slope of fEPSPs was recorded from field CA1 of hippocampal slices before and after delivery of TBS. LTP did not stabilize in slices prepared from 8-week-old CAG140 mice receiving bath infusions of heat-inactivated BDNF but appeared normal in slices from the same mutant mice (n = 4) treated with active BDNF (2 nM; P = 0.01).
Fig. 2.
Ampakine CX929 increases BDNF protein levels in hippocampus of CAG140 mice. (A) Representative Western blots show that mBDNF (14–15 kDa) levels are lower in hippocampus of 8-week-old CAG140 (HD) mice than in WT. This difference is eliminated in HD mice given CX929; the ampakine did not alter BDNF levels in WT mice. Samples from 2 mice per group are shown; nonadjacent lanes (separated by white space) were moved together for comparison purposes. Corresponding tubulin and actin immunobands from the stripped and reprobed blots are shown at the bottom of the lanes. (B) Densiometric analysis of Western blots confirmed that mBDNF levels are ≈30% lower in hippocampus of CAG140 mice relative to WT (*, P = 0.0007; group mean ± SEM raw band density values for n ≥ 8 per group). CAG140 mice treated with CX929 had higher mBDNF levels than those given vehicle (+, P = 0.008). Similar results were obtained when the values were normalized to those for the tubulin immunobands.
Fig. 3.
LTP deficits worsen with age in CAG140 mice and are reversed by in vivo CX929 treatment. (A) Plot of group mean (± SEM) fEPSP slopes recorded from hippocampal slices from 8-week-old CAG140 mice treated in vivo with vehicle or CX929. TBS elicited a comparable initial increase in synaptic responses in slices from WT and vehicle-treated CAG140 mice but responses decayed toward baseline in the latter group; percentage potentiation at the end of recording was significantly below that recorded from WTs (indicated by dashed line; P = 0.036). Administering CX929 in vivo rescued LTP in slices prepared 18 h after the last injection. (B) Graph shows group mean values for potentiation in slices from vehicle-treated WT and CAG140 mice at 8 and 16 weeks of age: LTP stabilization was more severely impaired in the latter group (numbers in bars = N per group; *, P = 0.036 and **, P = 0.007 compared with age-matched WT mice). (C) Representative fEPSP traces recorded during baseline (Left) or 30 min post-TBS (Right) in hippocampal slices from CAG140 mice treated with vehicle (Upper) or CX929 (Lower). Results are averages of 3 consecutive traces. Calibration: 1 mV, 10 ms. (D) Plot of fEPSP slopes in hippocampal slices from 16-week-old CAG140 mice treated with vehicle or CX929; percentage potentiation decayed to baseline >40 min in vehicle group (2.4 ± 6.2%) but was equivalent to WT in the CX929 group (46.4 ± 7.3% versus 50.6 ± 13.5%, respectively).
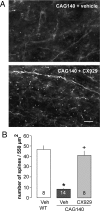
Fig. 4.
Up-regulating BDNF with ampakine CX929 rescues TBS-induced actin polymerization in dendritic spines. (A) Photomicrographs of post-TBS phalloidin labeling in CA1 stratum radiatum of hippocampal slices from CAG140 mice treated in vivo with vehicle or CX929. Densely labeled spines are more numerous in slices prepared from ampakine-treated mice. (Bar = 5 μm.) (B) The number of phalloidin-positive spines at 10 min post-TBS was significantly lower in slices prepared from vehicle-treated CAG140 mice than in those from vehicle-treated WT mice; CX929 treatment eliminated the mutant vs. WT difference. Values within bars denote number of slices analyzed per group. *, P = 0.0004 vs. WT; +, P = 0.004 vs. CAG140 vehicle group.
Fig. 5.
Locomotor deficits and behavioral changes in 16-week-old CAG140 mice with and without ampakine treatment. (A) Distance traveled during 5-min blocks of the 60-min test session on day 1. CAG140 mice given vehicle (n = 19) moved a shorter distance than WT mice (n = 17) on all 4 testing days (P < 0.0001; only day 1 shown). Treatment with CX929 (_n_ = 17) did not affect distance traveled in the mutants (_P_ < 0.005 compared with WT). Dashed horizontal line is the mean distance traveled by vehicle-treated WT mice per 5-min block for the 60-min test. (_B–D_) Plots show factors potentially contributing to the decrease in distance moved on day 1: move frequency (_B_), velocity (_C_), and duration (_D_). CAG140 mice decreased the frequency of their movements between the first and last 20 min of testing (_P_ ≤ 0.005) to a greater extent than WT mice (_P_ = 0.01) and made slower movements (_P_ = 0.00003); movement duration was unaffected. Ampakine treatment did not alter any of these measures. (_E_) Changes in the spatial distribution of movements from day 2 to day 3 (constant environment). (_Top_) WT mice consistently shifted their behavior between the 2 days, particularly in the refuge (R), open field (OF), and corners (C; *, _P_ ≤ 0.001). (_Middle_) Shifts in exploratory activity for vehicle-treated CAG140 mice were variable and did not reach statistical significance (_P_ > 0.8 for all 5 zones). (Bottom) However, CAG140 mice given CX929 were as consistent in their between-day changes as were WT mice (+, P ≤ 0.05 to 0.0001 for all 5 regions, 2-tailed t tests). P, porch; Ob, both objects.
Fig. 6.
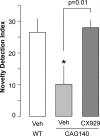
Ampakine CX929 reverses a long-term memory deficit in CAG140 mice. Novel object recognition was assessed by replacing 1 of 2 identical objects present on day 3 with an unfamiliar object on day 4. The time spent in the switch (new object) location was expressed as a fraction of time spent in both locations; the percentage increase (relative to day 4 values) in the day 3 to day 4 ratios was used as a novelty detection index. This index was smaller in vehicle-treated CAG140 mice (10 ± 26%; mean ± SD) than in WT mice (27 ± 17%; P = 0.02) and ampakine-treated CAG140 mice (28 ± 11%; P = 0.01).
Similar articles
- Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington's disease.
Lynch G, Kramar EA, Rex CS, Jia Y, Chappas D, Gall CM, Simmons DA. Lynch G, et al. J Neurosci. 2007 Apr 18;27(16):4424-34. doi: 10.1523/JNEUROSCI.5113-06.2007. J Neurosci. 2007. PMID: 17442827 Free PMC article. - Fingolimod (FTY720) enhances hippocampal synaptic plasticity and memory in Huntington's disease by preventing p75NTR up-regulation and astrocyte-mediated inflammation.
Miguez A, García-Díaz Barriga G, Brito V, Straccia M, Giralt A, Ginés S, Canals JM, Alberch J. Miguez A, et al. Hum Mol Genet. 2015 Sep 1;24(17):4958-70. doi: 10.1093/hmg/ddv218. Epub 2015 Jun 10. Hum Mol Genet. 2015. PMID: 26063761 - Modulation of AMPA receptor surface diffusion restores hippocampal plasticity and memory in Huntington's disease models.
Zhang H, Zhang C, Vincent J, Zala D, Benstaali C, Sainlos M, Grillo-Bosch D, Daburon S, Coussen F, Cho Y, David DJ, Saudou F, Humeau Y, Choquet D. Zhang H, et al. Nat Commun. 2018 Oct 15;9(1):4272. doi: 10.1038/s41467-018-06675-3. Nat Commun. 2018. PMID: 30323233 Free PMC article. - The substrates of memory: defects, treatments, and enhancement.
Lynch G, Rex CS, Chen LY, Gall CM. Lynch G, et al. Eur J Pharmacol. 2008 May 6;585(1):2-13. doi: 10.1016/j.ejphar.2007.11.082. Epub 2008 Mar 4. Eur J Pharmacol. 2008. PMID: 18374328 Free PMC article. Review.
Cited by
- Bidirectional Dysregulation of AMPA Receptor-Mediated Synaptic Transmission and Plasticity in Brain Disorders.
Zhang H, Bramham CR. Zhang H, et al. Front Synaptic Neurosci. 2020 Jul 10;12:26. doi: 10.3389/fnsyn.2020.00026. eCollection 2020. Front Synaptic Neurosci. 2020. PMID: 32754026 Free PMC article. Review. - Conditional BDNF release under pathological conditions improves Huntington's disease pathology by delaying neuronal dysfunction.
Giralt A, Carretón O, Lao-Peregrin C, Martín ED, Alberch J. Giralt A, et al. Mol Neurodegener. 2011 Oct 10;6(1):71. doi: 10.1186/1750-1326-6-71. Mol Neurodegener. 2011. PMID: 21985529 Free PMC article. - BDNF Therapeutic Mechanisms in Neuropsychiatric Disorders.
Bazzari AH, Bazzari FH. Bazzari AH, et al. Int J Mol Sci. 2022 Jul 29;23(15):8417. doi: 10.3390/ijms23158417. Int J Mol Sci. 2022. PMID: 35955546 Free PMC article. Review. - Interactions between Tumor Cells, Neurons, and Microglia in the Glioma Microenvironment.
Radin DP, Tsirka SE. Radin DP, et al. Int J Mol Sci. 2020 Nov 11;21(22):8476. doi: 10.3390/ijms21228476. Int J Mol Sci. 2020. PMID: 33187183 Free PMC article. Review. - Evidence linking microRNA suppression of essential prosurvival genes with hippocampal cell death after traumatic brain injury.
Boone DK, Weisz HA, Bi M, Falduto MT, Torres KEO, Willey HE, Volsko CM, Kumar AM, Micci MA, Dewitt DS, Prough DS, Hellmich HL. Boone DK, et al. Sci Rep. 2017 Jul 27;7(1):6645. doi: 10.1038/s41598-017-06341-6. Sci Rep. 2017. PMID: 28751711 Free PMC article.
References
- Group HsDCR. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. - PubMed
- Vonsattel J, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–384. - PubMed
- Ho AK, et al. Profile of cognitive progression in early Huntington's disease. Neurology. 2003;61:1702–1706. - PubMed
- Lawrence A, et al. Evidence for specific cognitive deficits in preclinical Huntington's disease. Brain Pathol. 1998;121:1329–1341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS051823/NS/NINDS NIH HHS/United States
- T32 AG000096/AG/NIA NIH HHS/United States
- NS051823/NS/NINDS NIH HHS/United States
- NS045260/NS/NINDS NIH HHS/United States
- P01 NS045260/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases