Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling - PubMed (original) (raw)
Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling
Adrian F Arechiga et al. J Immunol. 2009.
Abstract
PTPN22 is a gene encoding the protein tyrosine phosphatase Lyp. A missense mutation changing residue 1858 from cytosine to thymidine (1858C/T) is associated with multiple autoimmune disorders. Studies have demonstrated that Lyp has an inhibitory effect on TCR signaling; however, the presence of autoantibodies in all of the diseases associated with the 1858T variant and recent evidence that Ca(2+) flux is altered in B cells of 1858T carriers indicate a role for Lyp in B cell signaling. In this study we show that B cell signal transduction is impaired in individuals who express the variant. This defect in signaling is characterized by a deficit in proliferation, a decrease in phosphorylation of key signaling proteins, and is reversed by inhibition of Lyp. These findings suggest that the PTPN22 1858T variant alters BCR signaling and implicate B cells in the mechanism by which the PTPN22 1858T variant contributes to autoimmunity.
Figures
FIGURE 1
B cell proliferation is diminished in 1858C/T subjects. A, Total (CD19+; top), naive (CD19+CD27-; middle), and memory (CD19+CD27+; bottom) B cells were purified and activated with anti-IgG plus IgM F(ab′)2 (IgM+IgG) or PMA/ionomycin (P+I). Proliferation was measured at 72 h. Each dot represents a unique individual. B, B cells were activated as above and the CD27+ and CD27- populations were assessed by FACS for cleaved caspase-3 (n = 4 per group). Unt, Untreated.
FIGURE 2
Total phosphorylated tyrosine is significantly decreased in memory B cells of PTPN22 1858C/T subjects. Pan-tyrosine phosphorylation was assessed in B cells by intracellular flow cytometry. A, The percentage of unstimulated CD27+ (memory) and CD27- (naive) B cells with basal tyrosine phosphorylation is shown. B, B cells were activated for 2 min (2′) with anti-IgM/IgG. The log10-fold change in the mean fluorescence intensity of phosphorylation relative to the unstimulated control for CD27- naive and CD27+ memory B cells is shown. Black bars represent C/C samples (n = 6) and gray bars represent C/T samples (n = 6).
FIGURE 3
B cells from subjects harboring the 1858 T variant display BCR-mediated signaling defects. B cells were isolated and then activated with anti-IgM for the indicated time in minutes (′), followed by cell lysis. Western blotting was performed with the following Abs: phospho (P)-Akt-Ser473, phospho-PLC_γ_2-Tyr759, Syk, phospho-Syk-Tyr525/526, phospho-p38 MAPK-Thr180/Tyr182, and Lyp. A, Two control subjects were compared, one homozygous for 1858C/C and the other heterozygous for 1858C/T. B, Two control subjects were compared, one homozygous for 1858C/C and the other homozygous for the1858T/T variant. Blots are representative of three independent experiments. C, B cells were stimulated with soluble anti-IgM plus IgG F(ab′)2 for the indicated times. Total phospho-PLC_γ_2 (Tyr759) was assessed by intracellular flow cytometry in the CD27+ memory population of 1858 C/C and 1858 C/T subjects (n = 6). Mean fluorescence intensity (MFI) values for phospho-PLC_γ_2 are shown.
FIGURE 4
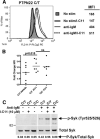
Inhibition of Lyp by I-C11 enhances B cell signaling in PTPN22 C/T subjects. A, Freshly isolated B cells were treated with vehicle or 5 _μ_M I-C11 followed by stimulation (stim) for 5 min with anti-IgM and intra-cellular staining for phospho-PLC_γ_2. B, Total B cells were treated as in A followed by stimulation with anti-kappa and intracellular staining for phospho-PLC_γ_2. Each dot represents the fold change in mean fluorescence intensity (MFI) relative to the unstimulated control for a unique individual. C, B cells were treated with vehicle or I-C11 (10 _μ_M) and stimulated as in A. Whole cell lysates were immunoblotted using anti-phospho-Syk (p-Syk; Tyr525/526) and then reprobed with anti-Syk antisera (lower panel). Band intensities were quantified by densitometry (ImageQuant TL software). The ratio of phospho-Syk (P-Syk) to total Syk protein is shown below each lane. Adjacent panels depicted in C are from the same gel. Data shown are representative of three experiments.
Similar articles
- Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant.
Habib T, Funk A, Rieck M, Brahmandam A, Dai X, Panigrahi AK, Luning Prak ET, Meyer-Bahlburg A, Sanda S, Greenbaum C, Rawlings DJ, Buckner JH. Habib T, et al. J Immunol. 2012 Jan 1;188(1):487-96. doi: 10.4049/jimmunol.1102176. Epub 2011 Nov 21. J Immunol. 2012. PMID: 22105996 Free PMC article. Clinical Trial. - Overexpression of the PTPN22 Autoimmune Risk Variant LYP-620W Fails to Restrain Human CD4+ T Cell Activation.
Perry DJ, Peters LD, Lakshmi PS, Zhang L, Han Z, Wasserfall CH, Mathews CE, Atkinson MA, Brusko TM. Perry DJ, et al. J Immunol. 2021 Aug 1;207(3):849-859. doi: 10.4049/jimmunol.2000708. Epub 2021 Jul 23. J Immunol. 2021. PMID: 34301848 Free PMC article. - Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes.
Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Rieck M, et al. J Immunol. 2007 Oct 1;179(7):4704-10. doi: 10.4049/jimmunol.179.7.4704. J Immunol. 2007. PMID: 17878369 - The putative role of the C1858T polymorphism of protein tyrosine phosphatase PTPN22 gene in autoimmunity.
Gianchecchi E, Palombi M, Fierabracci A. Gianchecchi E, et al. Autoimmun Rev. 2013 May;12(7):717-25. doi: 10.1016/j.autrev.2012.12.003. Epub 2012 Dec 20. Autoimmun Rev. 2013. PMID: 23261816 Review. - The role of PTPN22 risk variant in the development of autoimmunity: finding common ground between mouse and human.
Rawlings DJ, Dai X, Buckner JH. Rawlings DJ, et al. J Immunol. 2015 Apr 1;194(7):2977-84. doi: 10.4049/jimmunol.1403034. J Immunol. 2015. PMID: 25795788 Free PMC article. Review.
Cited by
- Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant.
Habib T, Funk A, Rieck M, Brahmandam A, Dai X, Panigrahi AK, Luning Prak ET, Meyer-Bahlburg A, Sanda S, Greenbaum C, Rawlings DJ, Buckner JH. Habib T, et al. J Immunol. 2012 Jan 1;188(1):487-96. doi: 10.4049/jimmunol.1102176. Epub 2011 Nov 21. J Immunol. 2012. PMID: 22105996 Free PMC article. Clinical Trial. - PTPN22 polymorphisms may indicate a role for this gene in atopic dermatitis in West Highland white terriers.
Roque JB, O'Leary CA, Kyaw-Tanner M, Duffy DL, Gharahkhani P, Vogelnest L, Mason K, Shipstone M. Roque JB, et al. BMC Res Notes. 2011 Dec 30;4:571. doi: 10.1186/1756-0500-4-571. BMC Res Notes. 2011. PMID: 22208456 Free PMC article. - Developments in the clinical understanding of lupus.
Crow MK. Crow MK. Arthritis Res Ther. 2009;11(5):245. doi: 10.1186/ar2762. Epub 2009 Oct 14. Arthritis Res Ther. 2009. PMID: 19849817 Free PMC article. Review. - Clinical characteristics and PTPN22 1858C/T variant analysis in Jordanian Arab vitiligo patients.
Alkhateeb A, Qarqaz F, Al-Sabah J, Al Rashaideh T. Alkhateeb A, et al. Mol Diagn Ther. 2010 Jun 1;14(3):179-84. doi: 10.1007/BF03256371. Mol Diagn Ther. 2010. PMID: 20560680 - Structure-activity relationship studies and design of a PTPN22 inhibitor with enhanced isozyme selectivity and cellular efficacy.
Jassim BA, Bai Y, Qu Z, Sander CJ, Lin J, Miao J, Zhang ZY. Jassim BA, et al. Eur J Med Chem. 2025 Feb 5;283:117129. doi: 10.1016/j.ejmech.2024.117129. Epub 2024 Dec 6. Eur J Med Chem. 2025. PMID: 39693863
References
- Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, Ardlie KG, Huang Q, Smith AM, Spoerke JM, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 2004;75:330–337. - PMC - PubMed
- Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, Donaldson PT, Ball SG, James RA, Quinton R, Perros P, Pearce SH. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves’ disease. J. Clin. Endocrinol. Metab. 2004;89:5862–5865. - PubMed
- Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JM, Vella A, Nutland S, Rance HE, Maier L, et al. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020–3023. - PubMed
- Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat. Genet. 2004;36:337–338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous