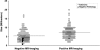Outcome of using the histological pseudocapsule as a surgical capsule in Cushing disease - PubMed (original) (raw)
Outcome of using the histological pseudocapsule as a surgical capsule in Cushing disease
Jay Jagannathan et al. J Neurosurg. 2009 Sep.
Abstract
Object: Many patients with Cushing disease still have active or recurrent disease after pituitary surgery. The histological pseudocapsule of a pituitary adenoma is a layer of compressed normal anterior lobe that surrounds the adenoma and can be used during surgery to identify and guide removal of the tumor. In this study the authors examined the results of using the pseudocapsule as a surgical capsule in the resection of adenomas in patients with Cushing disease.
Methods: The authors reviewed a prospective database of data obtained in patients with Cushing disease who underwent surgery. The analysis included all cases in which a lesion was identified during surgery and in which the lesion was believed to be confined to the pituitary gland in patients with Cushing disease between January 1990 and March 2007. Since the objective was to determine the success of using the pseudocapsule as a surgical capsule, patients with invasive tumors and patients in whom no lesion was identified during surgery-challenging cases for surgical success-were excluded from analysis.
Results: In 261 patients an encapsulated adenoma was identified at surgery. Tumor was visible on MR imaging in 135 patients (52%); in 126 patients (48%) MR imaging detected no tumor. The range of tumor size overlapped considerably in the groups with positive and negative MR imaging results, indicating that in addition to size other features of the adenoma influence the results of MR imaging. In 252 patients hypercortisolism resolved after the first operation, whereas in 9 patients (3 with positive MR imaging and 6 with negative MR imaging) early reoperation was required. Hypercortisolism resolved in all 261 patients (256 with hypocortisolism and 5 with eucortisolism) before hospital discharge. Forty-six patients (18%) had postoperative electrolyte abnormalities (30 with hyponatremia and 16 with diabetes insipidus), but only 2 patients required treatment at discharge. The mean clinical follow-up duration was 84 months (range 12-215 months). Six patients (2%) had recurrence of hypercortisolism, all of whom were treated successfully with reoperation.
Conclusions: Because of their small size, adenomas can be challenging to identify in patients with Cushing disease. Use of the histological pseudocapsule of an adenoma allows accurate identification of the tumor and helps guide its complete excision. With this approach the overall remission rate is high and the rate of complications is low.
Figures
Fig. 1
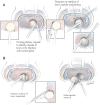
Illustrations of the surgical technique. A: A curvilinear incision is made through the pituitary capsule just beyond the point at which the most superficial dome of the tumor reaches the surface of the gland (left image). This permits a thin layer of normal gland to be traversed before reaching the surgical capsule of the adenoma (left inset), allowing easy identification of the surgical capsule and the creation of a surgical plane of dissection at the margin of the tumor, at the interface of the normal gland and the surgical capsule of the adenoma. This interface is further defined using the tips of the bipolar forceps in a series of movements parallel to the surface of the adenoma and in the crevice between the gland and adenoma (right image and center and right insets). Gentle dissection of the interface between the adenoma and gland is continued following the curvilinear margin of the adenoma. B: After the most superficial portion of the tumor has been defined circumferentially, the deeper adenoma margins are defined and dissected in a similar fashion; the posterior margin of the adenoma often requires dissection using a disc dissector and a small and/or medium ring curette (left image and inset). After the margins of the tumor have been completely dissected, to prevent rupture of the tumor the last remaining connection between the pseudocapsule of the specimen and the pituitary capsule is grasped with a small cup forceps and the tumor is removed (right image). In cases of tumors that are ≤ 8–10 mm in diameter, the entire tumor can usually be shelled out of its bed in the anterior lobe as an intact specimen. Successful and complete removal leaves a smoothly lined hemispherical tissue void in the anterior lobe. Modified from **J Neurosurg 104:**7–19, 2006.
Fig. 2
Graph showing disease in patients with negative and positive MR imaging. Mean tumor volume is depicted by the gray lines. Cases in which the encapsulated lesion removed at surgery proved to be an ACTH-staining adenoma are represented by open circles. Black circles represent patients in whom the encapsulated lesion removed at surgery did not contain an ACTH-staining adenoma. Most surgical specimens with negative pathology occurred in patients with negative MR imaging, and all were smaller than the mean size of those in the MR imaging–negative group.
Fig. 3
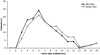
Graphic representation of tumor size on MR imaging (bold black line) compared with the tumor size at resection. Magnetic resonance imaging–based measurement was within 2 mm of the tumor size at surgery in most cases, although there was an overall tendency to underestimate tumor size on MR imaging, a factor that is probably related to the resection of the pseudocapsule with the adenoma.
Fig. 4
Graphic representation of tumor size in patients with negative MR imaging compared with those in patients with positive MR imaging (closed circles). The patients with negative MR imaging (open circles) tended to have smaller tumors, although there is considerable overlap in the range of tumor size with and without detection on MR imaging.
Fig. 5
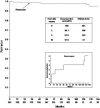
Kaplan-Meier plots showing the cumulative remission-free survival of Cushing disease. The numbers in the bottom-most row indicate the number of patients at risk at various intervals. Recurrences are shown in the lower inset.
Comment in
- Pituitary surgery. Editorial.
Weiss MH. Weiss MH. J Neurosurg. 2009 Sep;111(3):527-8; discussion 528-30. doi: 10.3171/2008.9.JNS081073. J Neurosurg. 2009. PMID: 19267525 No abstract available.
Similar articles
- Use of the histological pseudocapsule in surgery for Cushing disease: rapid postoperative cortisol decline predicting complete tumor resection.
Monteith SJ, Starke RM, Jane JA Jr, Oldfield EH. Monteith SJ, et al. J Neurosurg. 2012 Apr;116(4):721-7. doi: 10.3171/2011.12.JNS11886. Epub 2012 Jan 27. J Neurosurg. 2012. PMID: 22283193 - [Technical aspects and surgical strategy for removal of corticotroph pituitary adenoma].
Perrin G, Stevenaert A, Jouanneau E. Perrin G, et al. Neurochirurgie. 2002 May;48(2-3 Pt 2):186-214. Neurochirurgie. 2002. PMID: 12058125 Review. French. - Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors.
Oldfield EH, Vortmeyer AO. Oldfield EH, et al. J Neurosurg. 2006 Jan;104(1):7-19. doi: 10.3171/jns.2006.104.1.7. J Neurosurg. 2006. PMID: 16509142 - Pituitary magnetic resonance imaging findings do not influence surgical outcome in adrenocorticotropin-secreting microadenomas.
Salenave S, Gatta B, Pecheur S, San-Galli F, Visot A, Lasjaunias P, Roger P, Berge J, Young J, Tabarin A, Chanson P. Salenave S, et al. J Clin Endocrinol Metab. 2004 Jul;89(7):3371-6. doi: 10.1210/jc.2003-031908. J Clin Endocrinol Metab. 2004. PMID: 15240617 - [Clinical recurrence of Cushing syndrome without evidence of tumor recurrence: radical hypophysectomy?].
Estour B, Duthel R, Mounier Ch. Estour B, et al. Neurochirurgie. 2002 May;48(2-3 Pt 2):281-4. Neurochirurgie. 2002. PMID: 12058132 Review. French.
Cited by
- Acromegaly with negative pituitary MRI and no evidence of ectopic source: the role of transphenoidal pituitary exploration?
Daud S, Hamrahian AH, Weil RJ, Hamaty M, Prayson RA, Olansky L. Daud S, et al. Pituitary. 2011 Dec;14(4):414-7. doi: 10.1007/s11102-009-0205-z. Pituitary. 2011. PMID: 19904612 - Sex-related differences in Cushing's disease: a systematic review and meta-analysis.
Alqeeq BF, Ayyad M, Almadhoun WJ, Aboabdo M, Aldahdouh MS, Al-Tawil M, Al-Ghazali AM. Alqeeq BF, et al. Ann Saudi Med. 2024 Jan-Feb;44(1):55-65. doi: 10.5144/0256-4947.2024.55. Epub 2024 Feb 1. Ann Saudi Med. 2024. PMID: 38311874 Free PMC article. - Safety of transsphenoidal microsurgical approach in patients with an ACTH-secreting pituitary adenoma.
Donofrio CA, Losa M, Gemma M, Giudice L, Barzaghi LR, Mortini P. Donofrio CA, et al. Endocrine. 2017 Nov;58(2):303-311. doi: 10.1007/s12020-016-1214-0. Epub 2016 Dec 22. Endocrine. 2017. PMID: 28005257 - Development and Validation of a Prognostic Model for Post-Operative Recurrence of Pituitary Adenomas.
Lu L, Wan X, Xu Y, Chen J, Shu K, Lei T. Lu L, et al. Front Oncol. 2022 Apr 28;12:882049. doi: 10.3389/fonc.2022.882049. eCollection 2022. Front Oncol. 2022. PMID: 35574399 Free PMC article. - MRI-negative Cushing's Disease: Management Strategy and Outcomes in 15 Cases Utilizing a Pure Endoscopic Endonasal Approach.
Sharifi G, Amin AA, Sabahi M, Echeverry NB, Dilmaghani NA, Mousavinejad SA, Valizadeh M, Davoudi Z, Adada B, Borghei-Razavi H. Sharifi G, et al. BMC Endocr Disord. 2022 Jun 9;22(1):154. doi: 10.1186/s12902-022-01069-5. BMC Endocr Disord. 2022. PMID: 35676664 Free PMC article.
References
- Atkinson AB, Kennedy A, Wiggam MI, McCance DR, Sheridan B. Long-term remission rates after pituitary surgery for Cushing's disease: the need for long-term surveillance. Clin Endocrinol (Oxf) 2005;63:549–559. - PubMed
- Batista D, Courkoutsakis NA, Oldfield EH, Griffin KJ, Keil M, Patronas NJ, et al. Detection of adrenocorticotropin-secreting pituitary adenomas by magnetic resonance imaging in children and adolescents with cushing disease. J Clin Endocrinol Metab. 2005;90:5134–5140. - PubMed
- Blevins LS, Jr, Christy JH, Khajavi M, Tindall GT. Outcomes of therapy for Cushing's disease due to adrenocorticotropin-secreting pituitary macroadenomas. J Clin Endocrinol Metab. 1998;83:63–67. - PubMed
- Chee GH, Mathias DB, James RA, Kendall-Taylor P. Transsphenoidal pituitary surgery in Cushing's disease: can we predict outcome? Clin Endocrinol (Oxf) 2001;54:617–626. - PubMed
- Ciric I. Pituitary tumors. Neurol Clin. 1985;3:751–768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical