Quantification of intermediates formed during the reduction of nitrite by deoxyhemoglobin - PubMed (original) (raw)
Quantification of intermediates formed during the reduction of nitrite by deoxyhemoglobin
Maria T Salgado et al. J Biol Chem. 2009.
Abstract
Nitric oxide (NO) plays a crucial role in human physiology by regulating vascular tone and blood flow. The short life-span of NO in blood requires a mechanism to retain NO bioactivity in the circulation. Recent studies have suggested a mechanism involving the reduction of nitrite back to NO by deoxyhemoglobin in RBCs. A role for RBCs in transporting NO must, however, bypass the scavenging of NO in RBCs by hemoglobin. To understand how the nitrite reaction can deliver bioactive NO to the vasculature, we have studied the intermediates formed during the reaction. A reliable measure of the total concentration of heme-associated nitrite/NO intermediates formed was provided by combining filtration to measure free nitrite by chemiluminescence and electron paramagnetic resonance to measure the final product Hb(II)NO. By modifying the chemiluminescence method used to detect NO, we have been able to identify two intermediates: 1) a heme-associated nitrite complex that is released as NO in acid solution in the presence of ascorbate and 2) an intermediate that releases NO at neutral pH in the presence of ferricyanide when reacted with an Fe(III) ligand like azide. This species designated as "Hb(II)NO(+) (<--)(-->)Hb(III)NO" has properties of both isomeric forms resulting in a slower NO dissociation rate and much higher stability than Hb(III)NO, but provides a potential source for bioactive NO, which can be released from the RBC. This detailed analysis of the nitrite reaction with deoxyHb provides important insights into the mechanism for nitrite induced vasodilation by RBCs.
Figures
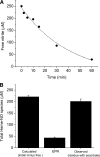
FIGURE 1.
Formation of heme-NO species during the reduction of nitrite (0.25 mm) by deoxyHb (1 mm) at 22 °C. A, consumption of nitrite as a function of reaction time. The amount of reacted nitrite was determined by the chemiluminescence method for measuring free nitrite (un-reacted nitrite) from the filtrate of the reaction mixture (see “Experimental Procedures” for details). B, the total amount of heme-NO species formed after 60 min calculated and observed by chemiluminescence and EPR. Calculated (initial minus free): these data correspond to the total amount of nitrite consumed during the reaction that is expected to have been converted to heme-NO species. This value was obtained by subtracting the amount of un-reacted nitrite at 60 min (A) from the initial amount of 250 μ
m
. EPR: these data correspond to the total amount of Hb(II)NO detected by EPR.Observed (residue with ascorbate): these data correspond to the total amount of heme-NO species detected in the residue by the chemiluminescence assay under acidic pH in the absence of sulfanilamide and the presence of ascorbate (see “Experimental Procedures” for details). Four injections were made for each sample. The average concentration from the integrated signals is shown. Values represent the mean ± S.D. of three independent experiments for each sample.
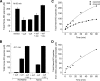
FIGURE 2.
The total amount of NO species determined by the chemiluminescence method at acidic pH under various experimental conditions when 0.25 mm nitrite is reacted with 1 mm deoxyHb up to 60 min at pH 7.4. A, total heme-NO species measured after 60 min of reaction time. The purge vessel contained 85% glacial acetic acid with 0.15
m
ferricyanide with or without sulfanilamide (100 m
m
) and/or ascorbate (62.5 m
m
). residue: sample with all free nitrite removed by filtration and ascorbate added to the purge vessel without sulfanilamide; the other samples involved the total reaction mixture with the additives indicated in the figure (see “Experimental Procedures” for additional details). B, total NO species measured after 1 min of reaction time. The purge vessel contained 85% glacial acetic acid with 0.15
m
ferricyanide with or without sulfanilamide (100 m
m
) and/or ascorbate (62.5 m
m
) as indicated in the figure. Results are shown for nitrite reacted with deoxyHb and for nitrite without hemoglobin under similar experimental conditions. C, comparison of the time course for the formation of the expected (•) and the observed (▪) total amount of heme-NO species. The expected total heme-NO species (•) was obtained by subtracting at each time point the free nitrite from the chemiluminescence obtained at acidic pH when ascorbate was present in the purge vessel without sulfanilamide. The observed total heme-NO species (▪) was obtained by the chemiluminescence assay at acidic pH with sulfanilamide and no ascorbate in the purge vessel. D, the time course for the formation of the nitrite-associated intermediate species. This time course is the difference spectrum between the expected and the observed time courses in C. Values represent the mean ± S.D. of three independent experiments for each sample at each time point.
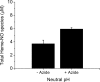
FIGURE 3.
The total amount of heme-NO species during the reaction of 0.25 mm nitrite with 1 mm deoxyHb up to 75 min at pH 7.4 that are detected without ascorbate reduction. A, the total amount of heme-NO species detected after 60 min reaction time by EPR and by chemiluminescence at acidic pH and neutral pH in the absence and presence of 1 m
m
sodium azide. Acidic pH: the purge vessel contained 100 m
m
sulfanilamide in 85% glacial acetic acid with 0.15
m
ferricyanide. Neutral pH: the purge vessel contained 0.2
m
ferricyanide in 50 m
m
phosphate buffer, pH 7.4 with or without 1 m
m
azide for a total volume of 8 ml. The 60-min reaction mixture was pretreated with 1 m
m
azide (s) or 1 m
m
azide was added in the purge vessel (v) (see “Experimental Procedures” for details). B, the time course for the formation of heme-NO species obtained by EPR (○); neutral pH without azide (•); neutral pH with azide (□); acidic pH (▪). C, time course for the formation of the azide released isomeric intermediate. The data were generated by subtracting the time course of the reaction mixture analyzed by the chemiluminescence assay under neutral pH conditions from the time course of the reaction analyzed under neutral pH conditions but in the presence of azide. Values represent the mean ± S.D. of three independent experiments for each sample at each time point.
FIGURE 4.
The total amount of heme-NO species detected by the chemiluminescence assay when 100 μm metHb is reacted with 100 μm NO for 60 min. The purge vessel contained 0.2
m
ferricyanide in 50 m
m
phosphate buffer, pH 7.4 with or without 1 m
m
azide for a total volume of 8 ml (see “Experimental Procedures” for details). Four injections were made for each sample, the average concentration from the integrated signals is shown. Values represent the mean ± S.D. of three independent experiments for each sample.
SCHEME 1.
The reaction scheme for the reduction of nitrite by deoxyhemoglobin.
Similar articles
- Red blood cell membrane-facilitated release of nitrite-derived nitric oxide bioactivity.
Salgado MT, Cao Z, Nagababu E, Mohanty JG, Rifkind JM. Salgado MT, et al. Biochemistry. 2015 Nov 10;54(44):6712-23. doi: 10.1021/acs.biochem.5b00643. Epub 2015 Oct 28. Biochemistry. 2015. PMID: 26478948 - A new paramagnetic intermediate formed during the reaction of nitrite with deoxyhemoglobin.
Salgado MT, Ramasamy S, Tsuneshige A, Manoharan PT, Rifkind JM. Salgado MT, et al. J Am Chem Soc. 2011 Aug 24;133(33):13010-22. doi: 10.1021/ja1115088. Epub 2011 Aug 2. J Am Chem Soc. 2011. PMID: 21755997 Free PMC article. - Intermediates detected by visible spectroscopy during the reaction of nitrite with deoxyhemoglobin: the effect of nitrite concentration and diphosphoglycerate.
Nagababu E, Ramasamy S, Rifkind JM. Nagababu E, et al. Biochemistry. 2007 Oct 16;46(41):11650-9. doi: 10.1021/bi700364e. Epub 2007 Sep 20. Biochemistry. 2007. PMID: 17880185 - Nitric oxide redox reactions and red cell biology.
Rifkind JM, Nagababu E, Ramasamy S. Rifkind JM, et al. Antioxid Redox Signal. 2006 Jul-Aug;8(7-8):1193-203. doi: 10.1089/ars.2006.8.1193. Antioxid Redox Signal. 2006. PMID: 16910767 Review. - The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase.
Gladwin MT, Grubina R, Doyle MP. Gladwin MT, et al. Acc Chem Res. 2009 Jan 20;42(1):157-67. doi: 10.1021/ar800089j. Acc Chem Res. 2009. PMID: 18783254 Review.
Cited by
- Glass matrix-facilitated thermal reduction: a tool for probing reactions of met hemoglobin with nitrite and nitric oxide.
Navati MS, Friedman JM. Navati MS, et al. J Phys Chem B. 2010 Mar 4;114(8):2938-43. doi: 10.1021/jp909425z. J Phys Chem B. 2010. PMID: 20146537 Free PMC article. - Reactivity of glass-embedded met hemoglobin derivatives toward external NO: implications for nitrite-mediated production of bioactive NO.
Navati MS, Friedman JM. Navati MS, et al. J Am Chem Soc. 2009 Sep 2;131(34):12273-9. doi: 10.1021/ja903364h. J Am Chem Soc. 2009. PMID: 19663497 Free PMC article. - The potential role of the red blood cell in nitrite-dependent regulation of blood flow.
Patel RP, Hogg N, Kim-Shapiro DB. Patel RP, et al. Cardiovasc Res. 2011 Feb 15;89(3):507-15. doi: 10.1093/cvr/cvq323. Epub 2010 Oct 14. Cardiovasc Res. 2011. PMID: 20952416 Free PMC article. Review. - Nitrite binding to globins: linkage isomerism, EPR silence and reductive chemistry.
Silaghi-Dumitrescu R, Svistunenko DA, Cioloboc D, Bischin C, Scurtu F, Cooper CE. Silaghi-Dumitrescu R, et al. Nitric Oxide. 2014 Nov 15;42:32-9. doi: 10.1016/j.niox.2014.08.007. Epub 2014 Aug 27. Nitric Oxide. 2014. PMID: 25172022 Free PMC article. - Routes for formation of S-nitrosothiols in blood.
Nagababu E, Rifkind JM. Nagababu E, et al. Cell Biochem Biophys. 2013 Nov;67(2):385-98. doi: 10.1007/s12013-011-9321-2. Cell Biochem Biophys. 2013. PMID: 22161622 Free PMC article. Review.
References
- Ignarro, L. J., Cirino, G., Casini, A., and Napoli, C. (1999) J. Cardiovasc. Pharmacol. 34 879–886 - PubMed
- Gautier, C., van, F. E., Mikula, I., Martasek, P., and Slama-Schwok, A. (2006) Biochem. Biophys. Res. Commun. 341 816–821 - PubMed
- Liu, X., Miller, M. J., Joshi, M. S., Sadowska-Krowicka, H., Clark, D. A., and Lancaster, J. R., Jr. (1998) J. Biol. Chem. 273 18709–18713 - PubMed
- Cosby, K., Partovi, K. S., Crawford, J. H., Patel, R. P., Reiter, C. D., Martyr, S., Yang, B. K., Waclawiw, M. A., Zalos, G., Xu, X., Huang, K. T., Shields, H., Kim-Shapiro, D. B., Schechter, A. N., Cannon, R. O., III, and Gladwin, M. T. (2003) Nat. Med. 9 1498–1505 - PubMed
- Nagababu, E., Ramasamy, S., Abernethy, D. R., and Rifkind, J. M. (2003) J. Biol. Chem. 278 46349–46356 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources