Influence of bone marrow-derived hematopoietic cells on the tumor response to radiotherapy: experimental models and clinical perspectives - PubMed (original) (raw)
Review
Influence of bone marrow-derived hematopoietic cells on the tumor response to radiotherapy: experimental models and clinical perspectives
G-One Ahn et al. Cell Cycle. 2009.
Abstract
In this review, we highlight some of recent studies underscoring the importance of the tumor microenvironment, especially the role of bone marrow-derived myeloid cells, in restoring tumor growth after irradiation. Myeloid cells are hematopoietic cells that give rise to monocytes and macrophages in the peripheral blood and tissues. These cells have been shown to be proangiogenic in tumors promoting tumor growth. We also discuss our previously unpublished results on the effect of irradiation on the tumor vasculature including pericyte and basement membrane coverage to the endothelium of tumor blood vessels. We summarize the clinical significance of these studies including the use of MMP-9 inhibitors, administering white blood cell boosters, or planning safety margin of tumor volumes, in order to improve overall clinical benefits in cancer patients treated with radiotherapy.
Figures
Figure 1
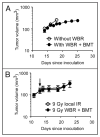
(A) Growth of MT1A2 tumor in FVB mice that did not receive whole body radiation (WBR) or bone marrow transplantation (BMT) (open circles) or in FVB mice that receive WBR at 9 Gy followed by BMT from Tie2lacZ transgenic mice (closed circles) 4 weeks prior. Tumors were implanted at the same time and monitored for their growth. (B) RIF tumor growth in female C3H mice that received 9 Gy local irradiation at the tumor implantation site (open circles) or in female C3H mice that received 9 Gy WBR followed by BMT from male C3H mice (closed circles) at the same time as the group in open circles. Tumors were implanted 4 weeks later, irradiated at approximately 200 mm3 with 20 Gy (arrow), and were monitored for their growth. Symbols in (A and B) are the mean ± s.e.m. for n = 5 per group.
Figure 2
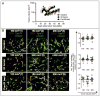
(A) Growth of MT1A2 tumors that were non-irradiated (control; open circles), irradiated and re-grown (IR tumor; closed circles), or grown in previously irradiated tissues (pre-IR bed; closed squares) in FVB mice. Arrowhead indicates local irradiation of 20 Gy to the ‘IR tumor group only. Numbers in red indicate points at which tumors were excised and examined histologically for basement membrane (B) and for pericytes (Fig. 3). Reproduced from with permission. (B) Left: Immunostaining of tumors in A for endothelial cells (CD31; red) or basement membrane (type IV collagen, green). Numbers in parentheses correspond to the numbers in red from (A). Right: The number of endothelial cells was subtracted from that of basement membrane to determine the presence of basement membrane empty sleeves (see the text). The difference in means between different volumes or treatment groups did not reach a statistical significance (p > 0.05).
Figure 3
Immunostaining of tumors from Figure 2 for endothelial cells (CD31; red) or pericytes (α-smooth muscle actin; α-SMA; green). Numbers in parentheses are as in Figure 2. Percentage of endothelial cells associated with pericytes were calculated and shown on right. Note that pre-IR tumors showed the percent endothelial cells with pericyte coverage to be significantly lower than other groups (p < 0.001).
Similar articles
- Myeloid cell trafficking and tumor angiogenesis.
Schmid MC, Varner JA. Schmid MC, et al. Cancer Lett. 2007 May 18;250(1):1-8. doi: 10.1016/j.canlet.2006.09.002. Epub 2006 Oct 17. Cancer Lett. 2007. PMID: 17049723 Free PMC article. Review. - Chapter 15. Methods to study myeloid cell roles in angiogenesis.
Schmid MC, Varner JA. Schmid MC, et al. Methods Enzymol. 2008;445:343-71. doi: 10.1016/S0076-6879(08)03015-2. Methods Enzymol. 2008. PMID: 19022067 - III. Angiogenesis: complexity of tumor vasculature and microenvironment.
Furuya M, Yonemitsu Y, Aoki I. Furuya M, et al. Curr Pharm Des. 2009;15(16):1854-67. doi: 10.2174/138161209788453275. Curr Pharm Des. 2009. PMID: 19519428 Review. - Role of myeloid cells in vascular endothelial growth factor-independent tumor angiogenesis.
Ferrara N. Ferrara N. Curr Opin Hematol. 2010 May;17(3):219-24. doi: 10.1097/MOH.0b013e3283386660. Curr Opin Hematol. 2010. PMID: 20308892 Review. - Decrease of VEGF-A in myeloid cells attenuates glioma progression and prolongs survival in an experimental glioma model.
Osterberg N, Ferrara N, Vacher J, Gaedicke S, Niedermann G, Weyerbrock A, Doostkam S, Schaefer HE, Plate KH, Machein MR. Osterberg N, et al. Neuro Oncol. 2016 Jul;18(7):939-49. doi: 10.1093/neuonc/now005. Epub 2016 Mar 6. Neuro Oncol. 2016. PMID: 26951383 Free PMC article.
Cited by
- Radiation-sensitive genetic prognostic model identifies individuals at risk for radiation resistance in head and neck squamous cell carcinoma.
You P, Liu S, Li Q, Xie D, Yao L, Guo C, Guo Z, Wang T, Qiu H, Guo Y, Li J, Zhou H. You P, et al. J Cancer Res Clin Oncol. 2023 Nov;149(17):15623-15640. doi: 10.1007/s00432-023-05304-x. Epub 2023 Sep 1. J Cancer Res Clin Oncol. 2023. PMID: 37656244 - Mechanical regulation of signal transduction in angiogenesis.
Flournoy J, Ashkanani S, Chen Y. Flournoy J, et al. Front Cell Dev Biol. 2022 Aug 19;10:933474. doi: 10.3389/fcell.2022.933474. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36081909 Free PMC article. Review. - Celecoxib Blocks Vasculogenic Mimicry via an Off-Target Effect to Radiosensitize Lung Cancer Cells: An Experimental Study.
Niu K, Chen XW, Qin Y, Zhang LP, Liao RX, Sun JG. Niu K, et al. Front Oncol. 2021 Sep 10;11:697227. doi: 10.3389/fonc.2021.697227. eCollection 2021. Front Oncol. 2021. PMID: 34568026 Free PMC article. - FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy.
Im JH, Buzzelli JN, Jones K, Franchini F, Gordon-Weeks A, Markelc B, Chen J, Kim J, Cao Y, Muschel RJ. Im JH, et al. Nat Commun. 2020 Aug 13;11(1):4064. doi: 10.1038/s41467-020-17914-x. Nat Commun. 2020. PMID: 32792542 Free PMC article. - Leukocytosis, prognosis biomarker in locally advanced head and neck cancer patients after chemoradiotherapy.
Schernberg A, Blanchard P, Chargari C, Ou D, Levy A, Gorphe P, Breuskin I, Atallah S, Caula A, Escande A, Janot F, Nguyen F, Temam S, Deutsch E, Tao Y. Schernberg A, et al. Clin Transl Radiat Oncol. 2018 Jul 6;12:8-15. doi: 10.1016/j.ctro.2018.07.002. eCollection 2018 Aug. Clin Transl Radiat Oncol. 2018. PMID: 30073209 Free PMC article.
References
- Verellen D, De Ridder M, Linthout N, Tournel K, Soete G, Storme G. Innovations in image-guided radiotherapy. Nat Rev Cancer. 2007;7:949–60. - PubMed
- Chao KSC, Ozyigit G, Tran BN, Cengiz M, Dempsey JF, Low DA. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Rad Oncol Biol Phys. 2003;55:312–21. - PubMed
- Eisbruch A, Marsh LH, Dawson LA, Bradford CR, Teknos TN, Chepeha DB, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Rad Oncol Biol Phys. 2004;59:28–42. - PubMed
- Chao KSC, Ozyigit G, Blanco AI, Thorstad WL, Deasy JO, Haughey BH, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: impact of tumor volume. Int J Rad Oncol Biol Phys. 2004;59:43–50. - PubMed
- Brown WT, Wu X, Fayad F, Fowler JF, Amendola BE, García S, et al. CyberKnife radiosurgery for stage I lung cancer: results at 36 months. Clin Lung Cancer. 2007;8:488–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA128873/CA/NCI NIH HHS/United States
- R01 CA118202/CA/NCI NIH HHS/United States
- R01 CA128873-02/CA/NCI NIH HHS/United States
- R01 CA118202-01A1/CA/NCI NIH HHS/United States
- CA128873/CA/NCI NIH HHS/United States
- CA118202/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous