Death receptor 5 internalization is required for lysosomal permeabilization by TRAIL in malignant liver cell lines - PubMed (original) (raw)
Comparative Study
. 2009 Jun;136(7):2365-2376.e1-7.
doi: 10.1053/j.gastro.2009.02.071. Epub 2009 Mar 9.
Justin L Mott, Steven F Bronk, Nathan W Werneburg, Alisan Kahraman, Maria Eugenia Guicciardi, Xue Wei Meng, Shigeru Kohno, Vijay H Shah, Scott H Kaufmann, Mark A McNiven, Gregory J Gores
Affiliations
- PMID: 19272388
- PMCID: PMC2693420
- DOI: 10.1053/j.gastro.2009.02.071
Comparative Study
Death receptor 5 internalization is required for lysosomal permeabilization by TRAIL in malignant liver cell lines
Yuko Akazawa et al. Gastroenterology. 2009 Jun.
Abstract
Background & aims: Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) cytotoxicity in hepatocellular carcinoma cells is mediated by lysosomal permeabilization. Our aims were to determine which TRAIL receptor, death receptor (DR) 4 or DR5, mediates lysosomal permeabilization and assess whether receptor endocytosis followed by trafficking to lysosomes contributes in this process.
Methods: TRAIL ligand internalization in Huh-7 cells was examined by confocal microscopy using Flag-tagged TRAIL, whereas DR4- and DR5-enhanced green fluorescent protein internalization was assessed by total internal reflection microscopy. Clathrin-dependent endocytosis was inhibited by expressing dominant negative dynamin.
Results: Although Huh-7 cells express both TRAIL receptors, short hairpin RNA silencing of DR5 but not DR4 attenuated TRAIL-mediated lysosomal permeabilization and apoptosis. The TRAIL/DR5 complex underwent rapid cellular internalization upon ligand stimulation, whereas the TRAIL/DR4 complex was not efficiently internalized. DR5-enhanced green fluorescent protein internalization was dependent on a dileucine-based internalization motif. Endocytosis of the TRAIL/DR5 complex was dynamin dependent and was required for rapid lysosomal permeabilization and apoptosis in multiple malignant hepatocellular and cholangiocarcinoma cell lines. Upon TRAIL treatment, DR5 colocalized with lysosomes after internalization. Inhibition of DR5 trafficking to lysosomes by Rab7 small interfering RNA also reduced TRAIL-mediated lysosomal disruption and apoptosis.
Conclusions: TRAIL-mediated endocytosis of DR5 with trafficking to lysosomes contributes to lysosomal protease release into the cytosol and efficient apoptosis in malignant liver cell lines.
Figures
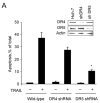
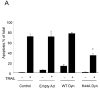
Figure 1. DR5 contributes more than DR4 to TRAIL-mediated lysosomal permeabilization and apoptosis
(A) Huh-7 cells stably transfected with short hairpin RNA (shRNA) complementary to DR4 or DR5, or untransfected Huh-7 (wild-type, wt), were treated with 4 ng/mL TRAIL for 8 hours. Apoptosis was assessed by morphological criteria after DAPI staining. Error bars in this and subsequent figures depict ± SEM from 3 or more independent experiments. * p<0.05, TRAIL-treated DR5-shRNA vs. wt. Insert, immunoblotting showing effective knockdown of DR4 and DR5 on total cell lysates. (B) After cells were treated with TRAIL for 8 hours, activity of activity of effector caspases 3 and 7 was measured by a fluorogenic assay. Data are expressed as fold-increase of relative fluorescence units (RFLU) over control value (untreated cells), which was arbitrarily set to 1, and represent the mean ± SE. * p<0.05, TRAIL treated DR5-shRNA vs. wt. (C) Huh-7 cells stably transfected with shRNA targeting DR4 or DR5, or untransfected Huh-7 (wild-type, wt), were treated with 4 ng/mL TRAIL for 4 hours. Localization of cathepsin B was visualized by immunofluorescence by using a confocal microscope (64x), and (D) cells were scored for punctate or diffuse appearance of the antigen. * p<0.05, TRAIL treated DR5-shRNA vs. wt. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
Figure 1. DR5 contributes more than DR4 to TRAIL-mediated lysosomal permeabilization and apoptosis
(A) Huh-7 cells stably transfected with short hairpin RNA (shRNA) complementary to DR4 or DR5, or untransfected Huh-7 (wild-type, wt), were treated with 4 ng/mL TRAIL for 8 hours. Apoptosis was assessed by morphological criteria after DAPI staining. Error bars in this and subsequent figures depict ± SEM from 3 or more independent experiments. * p<0.05, TRAIL-treated DR5-shRNA vs. wt. Insert, immunoblotting showing effective knockdown of DR4 and DR5 on total cell lysates. (B) After cells were treated with TRAIL for 8 hours, activity of activity of effector caspases 3 and 7 was measured by a fluorogenic assay. Data are expressed as fold-increase of relative fluorescence units (RFLU) over control value (untreated cells), which was arbitrarily set to 1, and represent the mean ± SE. * p<0.05, TRAIL treated DR5-shRNA vs. wt. (C) Huh-7 cells stably transfected with shRNA targeting DR4 or DR5, or untransfected Huh-7 (wild-type, wt), were treated with 4 ng/mL TRAIL for 4 hours. Localization of cathepsin B was visualized by immunofluorescence by using a confocal microscope (64x), and (D) cells were scored for punctate or diffuse appearance of the antigen. * p<0.05, TRAIL treated DR5-shRNA vs. wt. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
Figure 1. DR5 contributes more than DR4 to TRAIL-mediated lysosomal permeabilization and apoptosis
(A) Huh-7 cells stably transfected with short hairpin RNA (shRNA) complementary to DR4 or DR5, or untransfected Huh-7 (wild-type, wt), were treated with 4 ng/mL TRAIL for 8 hours. Apoptosis was assessed by morphological criteria after DAPI staining. Error bars in this and subsequent figures depict ± SEM from 3 or more independent experiments. * p<0.05, TRAIL-treated DR5-shRNA vs. wt. Insert, immunoblotting showing effective knockdown of DR4 and DR5 on total cell lysates. (B) After cells were treated with TRAIL for 8 hours, activity of activity of effector caspases 3 and 7 was measured by a fluorogenic assay. Data are expressed as fold-increase of relative fluorescence units (RFLU) over control value (untreated cells), which was arbitrarily set to 1, and represent the mean ± SE. * p<0.05, TRAIL treated DR5-shRNA vs. wt. (C) Huh-7 cells stably transfected with shRNA targeting DR4 or DR5, or untransfected Huh-7 (wild-type, wt), were treated with 4 ng/mL TRAIL for 4 hours. Localization of cathepsin B was visualized by immunofluorescence by using a confocal microscope (64x), and (D) cells were scored for punctate or diffuse appearance of the antigen. * p<0.05, TRAIL treated DR5-shRNA vs. wt. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
Figure 1. DR5 contributes more than DR4 to TRAIL-mediated lysosomal permeabilization and apoptosis
(A) Huh-7 cells stably transfected with short hairpin RNA (shRNA) complementary to DR4 or DR5, or untransfected Huh-7 (wild-type, wt), were treated with 4 ng/mL TRAIL for 8 hours. Apoptosis was assessed by morphological criteria after DAPI staining. Error bars in this and subsequent figures depict ± SEM from 3 or more independent experiments. * p<0.05, TRAIL-treated DR5-shRNA vs. wt. Insert, immunoblotting showing effective knockdown of DR4 and DR5 on total cell lysates. (B) After cells were treated with TRAIL for 8 hours, activity of activity of effector caspases 3 and 7 was measured by a fluorogenic assay. Data are expressed as fold-increase of relative fluorescence units (RFLU) over control value (untreated cells), which was arbitrarily set to 1, and represent the mean ± SE. * p<0.05, TRAIL treated DR5-shRNA vs. wt. (C) Huh-7 cells stably transfected with shRNA targeting DR4 or DR5, or untransfected Huh-7 (wild-type, wt), were treated with 4 ng/mL TRAIL for 4 hours. Localization of cathepsin B was visualized by immunofluorescence by using a confocal microscope (64x), and (D) cells were scored for punctate or diffuse appearance of the antigen. * p<0.05, TRAIL treated DR5-shRNA vs. wt. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
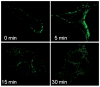
Figure 2. TRAIL undergoes cellular internalization upon ligand stimulation
(A) Huh-7 cells were incubated with FLAG-TRAIL cross-linked by anti-FLAG M2 antibody for 30 minutes on ice, followed by incubation in 37 °C for the indicated time. Cells were then fixed, incubated with fluorescent secondary antibody, and analyzed by confocal microscopy. (B) In order to visualize only internalized TRAIL, Huh-7 cells stably transfected with shRNA complementary to DR4 or DR5, or untransfected Huh-7 (wild-type, wt) were treated with FLAG-TRAIL and mouse monoclonal M2 antibody as illustrated in panel A, then washed with 0.2 M acetic acid before fixation to remove the membrane-associated FLAG-TRAIL. Cells were then incubated with fluorescent anti-mouse IgG. Blue fluorescence, DAPI staining; green fluorescence, internalized FLAG-TRAIL. (C) Quantification of internalized FLAG-TRAIL. Cells were treated as illustrated in panel B. Data were expressed as the area of the cell multiplied by the average fluorescence intensity in the cell above the background. * p<0.01, TRAIL treated DR5-shRNA vs. TRAIL treated wt. (D) Cells were transiently transfected with a plasmid expressing enhanced green fluorescent protein (EGFP)-tagged DR5 (DR5-EGFP). Cells were observed on a heated microscope stage at 37°C with or without 20 ng/mL of TRAIL for 30 minutes. Disappearance of membrane DR5 was observed by total internal reflection fluorescence (TIRF) microscopy. (E) The percentage receptor remaining on the cell surface was assessed by quantification of discrete fluorescent foci. * p<0.01, TRAIL-treated DR5-EGFP vs. DR4-EGFP.
Figure 2. TRAIL undergoes cellular internalization upon ligand stimulation
(A) Huh-7 cells were incubated with FLAG-TRAIL cross-linked by anti-FLAG M2 antibody for 30 minutes on ice, followed by incubation in 37 °C for the indicated time. Cells were then fixed, incubated with fluorescent secondary antibody, and analyzed by confocal microscopy. (B) In order to visualize only internalized TRAIL, Huh-7 cells stably transfected with shRNA complementary to DR4 or DR5, or untransfected Huh-7 (wild-type, wt) were treated with FLAG-TRAIL and mouse monoclonal M2 antibody as illustrated in panel A, then washed with 0.2 M acetic acid before fixation to remove the membrane-associated FLAG-TRAIL. Cells were then incubated with fluorescent anti-mouse IgG. Blue fluorescence, DAPI staining; green fluorescence, internalized FLAG-TRAIL. (C) Quantification of internalized FLAG-TRAIL. Cells were treated as illustrated in panel B. Data were expressed as the area of the cell multiplied by the average fluorescence intensity in the cell above the background. * p<0.01, TRAIL treated DR5-shRNA vs. TRAIL treated wt. (D) Cells were transiently transfected with a plasmid expressing enhanced green fluorescent protein (EGFP)-tagged DR5 (DR5-EGFP). Cells were observed on a heated microscope stage at 37°C with or without 20 ng/mL of TRAIL for 30 minutes. Disappearance of membrane DR5 was observed by total internal reflection fluorescence (TIRF) microscopy. (E) The percentage receptor remaining on the cell surface was assessed by quantification of discrete fluorescent foci. * p<0.01, TRAIL-treated DR5-EGFP vs. DR4-EGFP.
Figure 2. TRAIL undergoes cellular internalization upon ligand stimulation
(A) Huh-7 cells were incubated with FLAG-TRAIL cross-linked by anti-FLAG M2 antibody for 30 minutes on ice, followed by incubation in 37 °C for the indicated time. Cells were then fixed, incubated with fluorescent secondary antibody, and analyzed by confocal microscopy. (B) In order to visualize only internalized TRAIL, Huh-7 cells stably transfected with shRNA complementary to DR4 or DR5, or untransfected Huh-7 (wild-type, wt) were treated with FLAG-TRAIL and mouse monoclonal M2 antibody as illustrated in panel A, then washed with 0.2 M acetic acid before fixation to remove the membrane-associated FLAG-TRAIL. Cells were then incubated with fluorescent anti-mouse IgG. Blue fluorescence, DAPI staining; green fluorescence, internalized FLAG-TRAIL. (C) Quantification of internalized FLAG-TRAIL. Cells were treated as illustrated in panel B. Data were expressed as the area of the cell multiplied by the average fluorescence intensity in the cell above the background. * p<0.01, TRAIL treated DR5-shRNA vs. TRAIL treated wt. (D) Cells were transiently transfected with a plasmid expressing enhanced green fluorescent protein (EGFP)-tagged DR5 (DR5-EGFP). Cells were observed on a heated microscope stage at 37°C with or without 20 ng/mL of TRAIL for 30 minutes. Disappearance of membrane DR5 was observed by total internal reflection fluorescence (TIRF) microscopy. (E) The percentage receptor remaining on the cell surface was assessed by quantification of discrete fluorescent foci. * p<0.01, TRAIL-treated DR5-EGFP vs. DR4-EGFP.
Figure 2. TRAIL undergoes cellular internalization upon ligand stimulation
(A) Huh-7 cells were incubated with FLAG-TRAIL cross-linked by anti-FLAG M2 antibody for 30 minutes on ice, followed by incubation in 37 °C for the indicated time. Cells were then fixed, incubated with fluorescent secondary antibody, and analyzed by confocal microscopy. (B) In order to visualize only internalized TRAIL, Huh-7 cells stably transfected with shRNA complementary to DR4 or DR5, or untransfected Huh-7 (wild-type, wt) were treated with FLAG-TRAIL and mouse monoclonal M2 antibody as illustrated in panel A, then washed with 0.2 M acetic acid before fixation to remove the membrane-associated FLAG-TRAIL. Cells were then incubated with fluorescent anti-mouse IgG. Blue fluorescence, DAPI staining; green fluorescence, internalized FLAG-TRAIL. (C) Quantification of internalized FLAG-TRAIL. Cells were treated as illustrated in panel B. Data were expressed as the area of the cell multiplied by the average fluorescence intensity in the cell above the background. * p<0.01, TRAIL treated DR5-shRNA vs. TRAIL treated wt. (D) Cells were transiently transfected with a plasmid expressing enhanced green fluorescent protein (EGFP)-tagged DR5 (DR5-EGFP). Cells were observed on a heated microscope stage at 37°C with or without 20 ng/mL of TRAIL for 30 minutes. Disappearance of membrane DR5 was observed by total internal reflection fluorescence (TIRF) microscopy. (E) The percentage receptor remaining on the cell surface was assessed by quantification of discrete fluorescent foci. * p<0.01, TRAIL-treated DR5-EGFP vs. DR4-EGFP.
Figure 2. TRAIL undergoes cellular internalization upon ligand stimulation
(A) Huh-7 cells were incubated with FLAG-TRAIL cross-linked by anti-FLAG M2 antibody for 30 minutes on ice, followed by incubation in 37 °C for the indicated time. Cells were then fixed, incubated with fluorescent secondary antibody, and analyzed by confocal microscopy. (B) In order to visualize only internalized TRAIL, Huh-7 cells stably transfected with shRNA complementary to DR4 or DR5, or untransfected Huh-7 (wild-type, wt) were treated with FLAG-TRAIL and mouse monoclonal M2 antibody as illustrated in panel A, then washed with 0.2 M acetic acid before fixation to remove the membrane-associated FLAG-TRAIL. Cells were then incubated with fluorescent anti-mouse IgG. Blue fluorescence, DAPI staining; green fluorescence, internalized FLAG-TRAIL. (C) Quantification of internalized FLAG-TRAIL. Cells were treated as illustrated in panel B. Data were expressed as the area of the cell multiplied by the average fluorescence intensity in the cell above the background. * p<0.01, TRAIL treated DR5-shRNA vs. TRAIL treated wt. (D) Cells were transiently transfected with a plasmid expressing enhanced green fluorescent protein (EGFP)-tagged DR5 (DR5-EGFP). Cells were observed on a heated microscope stage at 37°C with or without 20 ng/mL of TRAIL for 30 minutes. Disappearance of membrane DR5 was observed by total internal reflection fluorescence (TIRF) microscopy. (E) The percentage receptor remaining on the cell surface was assessed by quantification of discrete fluorescent foci. * p<0.01, TRAIL-treated DR5-EGFP vs. DR4-EGFP.
Figure 3
Mutation of predicted internalization motif in DR5 blocks TRAIL mediated endocytosis of DR5. Huh-7 cells were transiently transfected with wild type (wt)- DR5-EGFP or mutant (mut)-DR5-EGFP for 48 hours. Cells were then treated with diluent or TRAIL (20 ng/mL) for 30 min at 37 °C on the heated stage of a TIRF microscope. The percentage of surface receptor remaining at 30 min imaged by TIRF was assessed by quantification of fluorescent particles. *p< 0.05, TRAIL treated wt-DR5-EGFP vs. TRAIL treated mut-DR5-EGFP.
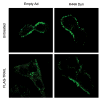
Figure 4
(A) Huh-7 cells were transduced with either adenovirus encoding K44A Dyn or empty vector (empty Ad). Twenty-four hours after infection, cells were treated with FLAG-TRAIL (400 ng/mL), fixed, stained with anti-FLAG antibody and analyzed by confocal microscopy. (B) Twenty-four hours after transient transfection with DR5-EGFP, cells were transduced with either K44A Dyn or empty adenovirus for 24 hours. Cells were then treated with diluent or TRAIL (20 ng/mL) for 30 min at 37 °C on the heated stage of a TIRF microscope. (C) The percentage of surface receptor remaining at 30 min imaged by TIRF was assessed by quantification of fluorescent particles. *p< 0.05, TRAIL treated empty ad vs. K44A Dyn ad.
Figure 4
(A) Huh-7 cells were transduced with either adenovirus encoding K44A Dyn or empty vector (empty Ad). Twenty-four hours after infection, cells were treated with FLAG-TRAIL (400 ng/mL), fixed, stained with anti-FLAG antibody and analyzed by confocal microscopy. (B) Twenty-four hours after transient transfection with DR5-EGFP, cells were transduced with either K44A Dyn or empty adenovirus for 24 hours. Cells were then treated with diluent or TRAIL (20 ng/mL) for 30 min at 37 °C on the heated stage of a TIRF microscope. (C) The percentage of surface receptor remaining at 30 min imaged by TIRF was assessed by quantification of fluorescent particles. *p< 0.05, TRAIL treated empty ad vs. K44A Dyn ad.
Figure 4
(A) Huh-7 cells were transduced with either adenovirus encoding K44A Dyn or empty vector (empty Ad). Twenty-four hours after infection, cells were treated with FLAG-TRAIL (400 ng/mL), fixed, stained with anti-FLAG antibody and analyzed by confocal microscopy. (B) Twenty-four hours after transient transfection with DR5-EGFP, cells were transduced with either K44A Dyn or empty adenovirus for 24 hours. Cells were then treated with diluent or TRAIL (20 ng/mL) for 30 min at 37 °C on the heated stage of a TIRF microscope. (C) The percentage of surface receptor remaining at 30 min imaged by TIRF was assessed by quantification of fluorescent particles. *p< 0.05, TRAIL treated empty ad vs. K44A Dyn ad.
Figure 5. K44A dynamin inhibits TRAIL-mediated cathepsin B release from lysosomes and cytotoxicity in Huh-7 cells
(A) Huh-7 cells infected with either wild type (WT) dynamin, K44A dynamin adenovirus, or empty adenovirus were incubated with TRAIL (4 ng/mL) for 8 hours. Apoptosis was assessed by morphological criteria after DAPI staining. Control: Not infected. * p<0.05, TRAIL-treated K44A Ad. vs. empty Ad. (B) Activation of effector caspases3 and 7 was measured by a fluorogenic assay. * p<0.05, TRAIL-treated K44A Ad. vs. empty Ad. (C) After cells were infected with adenovirus and treated with TRAIL as shown in Figure 5A, Annexin V study was performed using confocal microscopy. * p<0.01, TRAIL-treated K44A Ad. vs. empty Ad. (D) Beginning 24 h after transduction with K44A dynamin or empty adenovirus, Huh-7 cells were treated with TRAIL (4 ng/mL) for 4 hours. Cells were scored for punctate or diffuse appearance of cathepsin B as illustrated in Figure 2A. * p< 0. 05, TRAIL treated K44A Dyn Ad. vs. empty Ad. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
Figure 5. K44A dynamin inhibits TRAIL-mediated cathepsin B release from lysosomes and cytotoxicity in Huh-7 cells
(A) Huh-7 cells infected with either wild type (WT) dynamin, K44A dynamin adenovirus, or empty adenovirus were incubated with TRAIL (4 ng/mL) for 8 hours. Apoptosis was assessed by morphological criteria after DAPI staining. Control: Not infected. * p<0.05, TRAIL-treated K44A Ad. vs. empty Ad. (B) Activation of effector caspases3 and 7 was measured by a fluorogenic assay. * p<0.05, TRAIL-treated K44A Ad. vs. empty Ad. (C) After cells were infected with adenovirus and treated with TRAIL as shown in Figure 5A, Annexin V study was performed using confocal microscopy. * p<0.01, TRAIL-treated K44A Ad. vs. empty Ad. (D) Beginning 24 h after transduction with K44A dynamin or empty adenovirus, Huh-7 cells were treated with TRAIL (4 ng/mL) for 4 hours. Cells were scored for punctate or diffuse appearance of cathepsin B as illustrated in Figure 2A. * p< 0. 05, TRAIL treated K44A Dyn Ad. vs. empty Ad. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
Figure 5. K44A dynamin inhibits TRAIL-mediated cathepsin B release from lysosomes and cytotoxicity in Huh-7 cells
(A) Huh-7 cells infected with either wild type (WT) dynamin, K44A dynamin adenovirus, or empty adenovirus were incubated with TRAIL (4 ng/mL) for 8 hours. Apoptosis was assessed by morphological criteria after DAPI staining. Control: Not infected. * p<0.05, TRAIL-treated K44A Ad. vs. empty Ad. (B) Activation of effector caspases3 and 7 was measured by a fluorogenic assay. * p<0.05, TRAIL-treated K44A Ad. vs. empty Ad. (C) After cells were infected with adenovirus and treated with TRAIL as shown in Figure 5A, Annexin V study was performed using confocal microscopy. * p<0.01, TRAIL-treated K44A Ad. vs. empty Ad. (D) Beginning 24 h after transduction with K44A dynamin or empty adenovirus, Huh-7 cells were treated with TRAIL (4 ng/mL) for 4 hours. Cells were scored for punctate or diffuse appearance of cathepsin B as illustrated in Figure 2A. * p< 0. 05, TRAIL treated K44A Dyn Ad. vs. empty Ad. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
Figure 5. K44A dynamin inhibits TRAIL-mediated cathepsin B release from lysosomes and cytotoxicity in Huh-7 cells
(A) Huh-7 cells infected with either wild type (WT) dynamin, K44A dynamin adenovirus, or empty adenovirus were incubated with TRAIL (4 ng/mL) for 8 hours. Apoptosis was assessed by morphological criteria after DAPI staining. Control: Not infected. * p<0.05, TRAIL-treated K44A Ad. vs. empty Ad. (B) Activation of effector caspases3 and 7 was measured by a fluorogenic assay. * p<0.05, TRAIL-treated K44A Ad. vs. empty Ad. (C) After cells were infected with adenovirus and treated with TRAIL as shown in Figure 5A, Annexin V study was performed using confocal microscopy. * p<0.01, TRAIL-treated K44A Ad. vs. empty Ad. (D) Beginning 24 h after transduction with K44A dynamin or empty adenovirus, Huh-7 cells were treated with TRAIL (4 ng/mL) for 4 hours. Cells were scored for punctate or diffuse appearance of cathepsin B as illustrated in Figure 2A. * p< 0. 05, TRAIL treated K44A Dyn Ad. vs. empty Ad. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
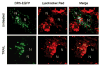
Figure 6. DR5 becomes localized to the lysosomes upon TRAIL-treatment
(A) Huh-7 cells were transfected with DR5-EGFP for 48 hours, then treated with 20 ng/mL TRAIL for 1 hour. Lysotracker Red was added 30 minutes before cells were observed by confocal microscopy. (B) Co-localization is calculated as ratio of co-localized DR5-EGFP and Lysotracker Red compared to total EGFP. * p<0.05, untreated Huh-7 cells vs. TRAIL treated Huh-7 cells. (C) After cells were treated with diluent or 20 ng/mL TRAIL for 1 hour, lysosomes were isolated. Collected lysosomes and whole-cell lysates were subjected to immunoblot analysis for indicated proteins. The purity of the lysosomal preparation was verified by the absence of the ER (calnexin), early endosome (EEA-1), and mitochondrial (cytochrome c oxidase) markers. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
Figure 6. DR5 becomes localized to the lysosomes upon TRAIL-treatment
(A) Huh-7 cells were transfected with DR5-EGFP for 48 hours, then treated with 20 ng/mL TRAIL for 1 hour. Lysotracker Red was added 30 minutes before cells were observed by confocal microscopy. (B) Co-localization is calculated as ratio of co-localized DR5-EGFP and Lysotracker Red compared to total EGFP. * p<0.05, untreated Huh-7 cells vs. TRAIL treated Huh-7 cells. (C) After cells were treated with diluent or 20 ng/mL TRAIL for 1 hour, lysosomes were isolated. Collected lysosomes and whole-cell lysates were subjected to immunoblot analysis for indicated proteins. The purity of the lysosomal preparation was verified by the absence of the ER (calnexin), early endosome (EEA-1), and mitochondrial (cytochrome c oxidase) markers. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
Figure 6. DR5 becomes localized to the lysosomes upon TRAIL-treatment
(A) Huh-7 cells were transfected with DR5-EGFP for 48 hours, then treated with 20 ng/mL TRAIL for 1 hour. Lysotracker Red was added 30 minutes before cells were observed by confocal microscopy. (B) Co-localization is calculated as ratio of co-localized DR5-EGFP and Lysotracker Red compared to total EGFP. * p<0.05, untreated Huh-7 cells vs. TRAIL treated Huh-7 cells. (C) After cells were treated with diluent or 20 ng/mL TRAIL for 1 hour, lysosomes were isolated. Collected lysosomes and whole-cell lysates were subjected to immunoblot analysis for indicated proteins. The purity of the lysosomal preparation was verified by the absence of the ER (calnexin), early endosome (EEA-1), and mitochondrial (cytochrome c oxidase) markers. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
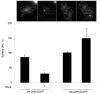
Figure 7. Rab7 is required for lysosomal trafficking of DR5 and apoptosis mediated by TRAIL
(A) Huh-7 cells were transfected with Rab7 siRNA or scrambled siRNA for 48 hours. Cells were lysed and were subjected to immunoblot analysis for Rab7, cathepsin B, and cathepsin D. (B) Huh-7 cells were transfected with Rab7 siRNA. After 24 hours, cells were transfected with DR5-EGFP for another 24 hours. Cells were then treated with 4 ng/mL TRAIL for 60 minutes. Lysotracker Red was added 30 minutes before cells were observed by confocal microscopy. Co-localization was calculated as a ratio of co-localized DR5-EGFP and Lysotracker Red compared to total EGFP. *p<0.05, TRAIL-treated Rab7 siRNA vs. control. (C) Untransfected (control) and Huh-7 cells transfected with Rab7 siRNA or scrambled siRNA were treated with TRAIL for 8 hours. Apoptosis was assessed by nuclear morphologic changes using nuclear binding dye DAPI. (D) Untransfected (control) and Huh-7 cells transfected with Rab7 siRNA or scrambled siRNA were treated with TRAIL for 4 hours. Cellular compartmentation of cathepsin D was assesed by immunofluorescence and confocal microscope. Cells were scored as punctate or diffuse based on cellular localization of the antigen * p< 0.05, TRAIL-treated Rab7 siRNA transfected cells vs. scrambled siRNA transfected cells. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
Figure 7. Rab7 is required for lysosomal trafficking of DR5 and apoptosis mediated by TRAIL
(A) Huh-7 cells were transfected with Rab7 siRNA or scrambled siRNA for 48 hours. Cells were lysed and were subjected to immunoblot analysis for Rab7, cathepsin B, and cathepsin D. (B) Huh-7 cells were transfected with Rab7 siRNA. After 24 hours, cells were transfected with DR5-EGFP for another 24 hours. Cells were then treated with 4 ng/mL TRAIL for 60 minutes. Lysotracker Red was added 30 minutes before cells were observed by confocal microscopy. Co-localization was calculated as a ratio of co-localized DR5-EGFP and Lysotracker Red compared to total EGFP. *p<0.05, TRAIL-treated Rab7 siRNA vs. control. (C) Untransfected (control) and Huh-7 cells transfected with Rab7 siRNA or scrambled siRNA were treated with TRAIL for 8 hours. Apoptosis was assessed by nuclear morphologic changes using nuclear binding dye DAPI. (D) Untransfected (control) and Huh-7 cells transfected with Rab7 siRNA or scrambled siRNA were treated with TRAIL for 4 hours. Cellular compartmentation of cathepsin D was assesed by immunofluorescence and confocal microscope. Cells were scored as punctate or diffuse based on cellular localization of the antigen * p< 0.05, TRAIL-treated Rab7 siRNA transfected cells vs. scrambled siRNA transfected cells. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
Figure 7. Rab7 is required for lysosomal trafficking of DR5 and apoptosis mediated by TRAIL
(A) Huh-7 cells were transfected with Rab7 siRNA or scrambled siRNA for 48 hours. Cells were lysed and were subjected to immunoblot analysis for Rab7, cathepsin B, and cathepsin D. (B) Huh-7 cells were transfected with Rab7 siRNA. After 24 hours, cells were transfected with DR5-EGFP for another 24 hours. Cells were then treated with 4 ng/mL TRAIL for 60 minutes. Lysotracker Red was added 30 minutes before cells were observed by confocal microscopy. Co-localization was calculated as a ratio of co-localized DR5-EGFP and Lysotracker Red compared to total EGFP. *p<0.05, TRAIL-treated Rab7 siRNA vs. control. (C) Untransfected (control) and Huh-7 cells transfected with Rab7 siRNA or scrambled siRNA were treated with TRAIL for 8 hours. Apoptosis was assessed by nuclear morphologic changes using nuclear binding dye DAPI. (D) Untransfected (control) and Huh-7 cells transfected with Rab7 siRNA or scrambled siRNA were treated with TRAIL for 4 hours. Cellular compartmentation of cathepsin D was assesed by immunofluorescence and confocal microscope. Cells were scored as punctate or diffuse based on cellular localization of the antigen * p< 0.05, TRAIL-treated Rab7 siRNA transfected cells vs. scrambled siRNA transfected cells. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
Figure 7. Rab7 is required for lysosomal trafficking of DR5 and apoptosis mediated by TRAIL
(A) Huh-7 cells were transfected with Rab7 siRNA or scrambled siRNA for 48 hours. Cells were lysed and were subjected to immunoblot analysis for Rab7, cathepsin B, and cathepsin D. (B) Huh-7 cells were transfected with Rab7 siRNA. After 24 hours, cells were transfected with DR5-EGFP for another 24 hours. Cells were then treated with 4 ng/mL TRAIL for 60 minutes. Lysotracker Red was added 30 minutes before cells were observed by confocal microscopy. Co-localization was calculated as a ratio of co-localized DR5-EGFP and Lysotracker Red compared to total EGFP. *p<0.05, TRAIL-treated Rab7 siRNA vs. control. (C) Untransfected (control) and Huh-7 cells transfected with Rab7 siRNA or scrambled siRNA were treated with TRAIL for 8 hours. Apoptosis was assessed by nuclear morphologic changes using nuclear binding dye DAPI. (D) Untransfected (control) and Huh-7 cells transfected with Rab7 siRNA or scrambled siRNA were treated with TRAIL for 4 hours. Cellular compartmentation of cathepsin D was assesed by immunofluorescence and confocal microscope. Cells were scored as punctate or diffuse based on cellular localization of the antigen * p< 0.05, TRAIL-treated Rab7 siRNA transfected cells vs. scrambled siRNA transfected cells. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
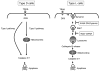
Figure 8. Schematic representation of the proposed model for TRAIL-induced apoptosis through internalization of TRAIL ligand-complex and lysosomal permeabilization
See text for details.
Similar articles
- Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein-induced lysosomal translocation of proapoptotic effectors is mediated by phosphofurin acidic cluster sorting protein-2 (PACS-2).
Werneburg NW, Bronk SF, Guicciardi ME, Thomas L, Dikeakos JD, Thomas G, Gores GJ. Werneburg NW, et al. J Biol Chem. 2012 Jul 13;287(29):24427-37. doi: 10.1074/jbc.M112.342238. Epub 2012 May 29. J Biol Chem. 2012. PMID: 22645134 Free PMC article. - TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5.
Zhang Y, Zhang B. Zhang Y, et al. Mol Cancer Res. 2008 Dec;6(12):1861-71. doi: 10.1158/1541-7786.MCR-08-0313. Mol Cancer Res. 2008. PMID: 19074831 - Combination of TRAIL with bortezomib shifted apoptotic signaling from DR4 to DR5 death receptor by selective internalization and degradation of DR4.
Bychkov ML, Gasparian ME, Dolgikh DA, Kirpichnikov MP. Bychkov ML, et al. PLoS One. 2014 Oct 13;9(10):e109756. doi: 10.1371/journal.pone.0109756. eCollection 2014. PLoS One. 2014. PMID: 25310712 Free PMC article. - Implication of Vegetable Oil-Derived Hydroxynonenal in the Lysosomal Cell Death for Lifestyle-Related Diseases.
Yamashima T. Yamashima T. Nutrients. 2023 Jan 24;15(3):609. doi: 10.3390/nu15030609. Nutrients. 2023. PMID: 36771317 Free PMC article. Review. - The Exploitation of Lysosomes in Cancer Therapy with Graphene-Based Nanomaterials.
Ristic B, Bosnjak M, Misirkic Marjanovic M, Stevanovic D, Janjetovic K, Harhaji-Trajkovic L. Ristic B, et al. Pharmaceutics. 2023 Jun 28;15(7):1846. doi: 10.3390/pharmaceutics15071846. Pharmaceutics. 2023. PMID: 37514033 Free PMC article. Review.
Cited by
- Synthetic ligands of death receptor 5 display a cell-selective agonistic effect at different oligomerization levels.
Beyrath J, Chekkat N, Smulski CR, Lombardo CM, Lechner MC, Seguin C, Decossas M, Spanedda MV, Frisch B, Guichard G, Fournel S. Beyrath J, et al. Oncotarget. 2016 Oct 4;7(40):64942-64956. doi: 10.18632/oncotarget.10508. Oncotarget. 2016. PMID: 27409341 Free PMC article. - Death receptor 5 signaling promotes hepatocyte lipoapoptosis.
Cazanave SC, Mott JL, Bronk SF, Werneburg NW, Fingas CD, Meng XW, Finnberg N, El-Deiry WS, Kaufmann SH, Gores GJ. Cazanave SC, et al. J Biol Chem. 2011 Nov 11;286(45):39336-48. doi: 10.1074/jbc.M111.280420. Epub 2011 Sep 22. J Biol Chem. 2011. PMID: 21941003 Free PMC article. - Hepatocyte death: a clear and present danger.
Malhi H, Guicciardi ME, Gores GJ. Malhi H, et al. Physiol Rev. 2010 Jul;90(3):1165-94. doi: 10.1152/physrev.00061.2009. Physiol Rev. 2010. PMID: 20664081 Free PMC article. Review. - GLI3-dependent repression of DR4 mediates hedgehog antagonism of TRAIL-induced apoptosis.
Kurita S, Mott JL, Almada LL, Bronk SF, Werneburg NW, Sun SY, Roberts LR, Fernandez-Zapico ME, Gores GJ. Kurita S, et al. Oncogene. 2010 Aug 26;29(34):4848-58. doi: 10.1038/onc.2010.235. Epub 2010 Jun 21. Oncogene. 2010. PMID: 20562908 Free PMC article. - Importin β1 protein-mediated nuclear localization of death receptor 5 (DR5) limits DR5/tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced cell death of human tumor cells.
Kojima Y, Nakayama M, Nishina T, Nakano H, Koyanagi M, Takeda K, Okumura K, Yagita H. Kojima Y, et al. J Biol Chem. 2011 Dec 16;286(50):43383-93. doi: 10.1074/jbc.M111.309377. Epub 2011 Oct 21. J Biol Chem. 2011. PMID: 22020938 Free PMC article.
References
- Kimberley FC, Screaton GR. Following a TRAIL: update on a ligand and its five receptors. Cell Res. 2004;14:359–72. - PubMed
- MacFarlane M, Inoue S, Kohlhaas SL, et al. Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death Differ. 2005;12:773–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK63947/DK/NIDDK NIH HHS/United States
- R01 HL086990/HL/NHLBI NIH HHS/United States
- DK 9875/DK/NIDDK NIH HHS/United States
- CA69008/CA/NCI NIH HHS/United States
- R01 DK063947-06/DK/NIDDK NIH HHS/United States
- HL86990/HL/NHLBI NIH HHS/United States
- R01 CA069008/CA/NCI NIH HHS/United States
- R37 DK044650/DK/NIDDK NIH HHS/United States
- R01 DK044650/DK/NIDDK NIH HHS/United States
- DK 44650/DK/NIDDK NIH HHS/United States
- R01 DK063947/DK/NIDDK NIH HHS/United States
- R01 DK059615/DK/NIDDK NIH HHS/United States
- F32 DK009875/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources