Cognitive deficits in interleukin-10-deficient mice after peripheral injection of lipopolysaccharide - PubMed (original) (raw)
Cognitive deficits in interleukin-10-deficient mice after peripheral injection of lipopolysaccharide
Amy F Richwine et al. Brain Behav Immun. 2009 Aug.
Abstract
Interleukin (IL)-10 is important for regulating inflammation but whether it protects against infection-related deficits in cognitive function is unknown. Therefore, the current study evaluated sickness behavior, hippocampal-dependent matching-to-place performance and several inflammatory cytokines and neurotrophins in wild-type (IL-10(+/+)) and IL-10-deficient (IL-10(-/-)) mice after i.p. injection of lipopolysaccharide (LPS). Additionally, morphology of dendrites of pyramidal neurons in the dorsal CA1 hippocampus was assessed. Treatment with LPS increased IL-1beta, IL-6, and tumor necrosis factor alpha (TNFalpha) mRNA in all brain areas examined including the hippocampus, in both IL-10(+/+) and IL-10(-/-) mice but the increase was largest in IL-10(-/-) mice. Plasma IL-1beta, IL-6 and TNFalpha were also higher in IL-10(-/-) mice compared to IL-10(+/+) mice after LPS. Consistent with increased inflammatory cytokines in IL-10(-/-) mice after LPS treatment, were a more lengthy sickness behavior syndrome and a more prominent reduction in hippocampal levels of nerve growth factor mRNA; brain-derived neurotrophic factor mRNA was reduced similarly in both genotypes after LPS. In a test of hippocampal-dependent learning and memory that required mice to integrate new information with previously learned information and switch strategies to master a task, IL-10(-/-) mice were found to be less efficient after LPS than were similarly treated wild-type mice. LPS did not affect morphology of dendrites of pyramidal neurons in the dorsal CA1 hippocampus in either genotype. Taken together the results are interpreted to suggest that during peripheral infection IL-10 inhibits sickness behavior and tribulations in hippocampal-dependent working memory via its propensity to mitigate inflammation. We conclude that IL-10 is critical for maintaining normal neuro-immune communication during infection.
Figures
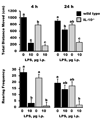
Figure 1
Locomotor behavior in IL-10+/+ (wild type) and IL-10−/− mice 4 and 24 h after a peripheral injection of saline or lipopolysaccharide (LPS). Bars are means ± SEM. Two-way ANOVA, with post-injection time as a repeated measure, revealed significant main effects (p < 0.001) of genotype and treatment for total distance moved. Only treatment was significant for rearing frequency (p < 0.001). No genotype × treatment interactions were present. For each graph, treatment means with dissimilar letters are significantly different (p < 0.05).
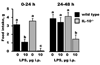
Figure 2
Food intake in IL-10+/+ (wild type) and IL-10−/− mice during 24 h periods after a peripheral injection of saline or lipopolysaccharide (LPS). Bars are means ± SEM. Two-way ANOVA revealed a significant main effect of treatment (p < 0.01) and a genotype × treatment interaction (p < 0.05) during both post-injection feeding periods. For each graph, treatment means with dissimilar letters are significantly different (p < 0.05).
Figure 3
Plasma cytokine concentrations in IL-10+/+ (wild type) and IL-10−/− mice 4 h after a peripheral injection of lipopolysaccharide (LPS). Bars are means ± SEM. Plasma cytokine concentrations in mice receiving a peripheral saline injection were below the detectable threshold and were therefore excluded from the statistical analysis. One-way ANOVA revealed significant genotype effects for IL-1β (p < 0.05), IL-10 (p < 0.05), TNFα (p < 0.01), and IL-6 (p < 0.001). For each graph, treatment means with dissimilar letters are significantly different (p < 0.05).
Figure 4
Plasma corticosterone concentrations in IL-10+/+ (wild type) and IL-10−/− mice 4, 24, and 72 h after a peripheral injection of saline or lipopolysaccharide (LPS). Bars are means ± SEM. Two-way ANOVA revealed a significant main effect of treatment at 4 (p < 0.001), 24 (p < 0.05), and 72 h (p < 0.05). A significant main effect of genotype (p < 0.01) and a genotype × treatment interaction (p < 0.001) were observed 24 h post-injection. For each graph, treatment means with dissimilar letters are significantly different (p < 0.05).
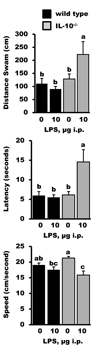
Figure 5
Performance of IL-10+/+ (wild type) and IL-10−/− mice in a matching-to-place paradigm 24 h after a peripheral injection of saline or lipopolysaccharide (LPS). Bars are test session means ± SEM. The main effect of genotype was observed for distance swam (p < 0.01) and latency (p < 0.01), while the main effect of treatment was observed for latency (p < 0.05) and speed (p < 0.001). Significant genotype × treatment interactions (p < 0.05) were observed for all three measures. For each graph, treatment means with dissimilar letters are significantly different (p < 0.05).
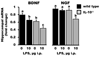
Figure 6
Hippocampal expression of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in IL-10+/+ (wild type) and IL-10−/− mice after a peripheral injection of saline or lipopolysaccharide (LPS). Neurotrophin mRNA expression was determined 4, 24, and 72 h post-injection, but no effect of time was evident so data were pooled across time. Bars are means ± SEM. Significant main effects (p < 0.001) of genotype and treatment were observed for both BDNF and NGF. Additionally, a genotype × treatment interaction (p < 0.001) was observed for NGF. For each graph, treatment means with dissimilar letters are significantly different (p <0.05).
Figure 7
Dendritic complexity of pyramidal hippocampal neurons in the dorsal CA1 region. The total number of neuronal intersections were measured by the Sholl Ring method in basal and apical trees in IL-10+/+ (wild type) and IL-10−/− mice 72 h after a peripheral injection of saline or lipopolysaccharide (LPS). Bars are means ± SEM. Two-way ANOVA of the number of intersections revealed a significant main effect of genotype on the basal tree (p < 0.05). No other significant effects were observed.
Similar articles
- Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation.
Krzyszton CP, Sparkman NL, Grant RW, Buchanan JB, Broussard SR, Woods J, Johnson RW. Krzyszton CP, et al. Am J Physiol Regul Integr Comp Physiol. 2008 Oct;295(4):R1109-14. doi: 10.1152/ajpregu.90302.2008. Epub 2008 Jul 23. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18650318 Free PMC article. - Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation.
Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham JA, Juraska JM, Johnson RW. Richwine AF, et al. Psychoneuroendocrinology. 2008 Nov;33(10):1369-77. doi: 10.1016/j.psyneuen.2008.08.003. Epub 2008 Sep 20. Psychoneuroendocrinology. 2008. PMID: 18805643 - Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers.
Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Sparkman NL, et al. J Neurosci. 2006 Oct 18;26(42):10709-16. doi: 10.1523/JNEUROSCI.3376-06.2006. J Neurosci. 2006. PMID: 17050710 Free PMC article. - Minocycline protects against lipopolysaccharide-induced cognitive impairment in mice.
Hou Y, Xie G, Liu X, Li G, Jia C, Xu J, Wang B. Hou Y, et al. Psychopharmacology (Berl). 2016 Mar;233(5):905-16. doi: 10.1007/s00213-015-4169-6. Epub 2015 Dec 8. Psychopharmacology (Berl). 2016. PMID: 26645224 - Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system.
Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Chen J, et al. Brain Behav Immun. 2008 Mar;22(3):301-11. doi: 10.1016/j.bbi.2007.08.014. Epub 2007 Oct 24. Brain Behav Immun. 2008. PMID: 17951027 Free PMC article.
Cited by
- Altered KLOTHO and NF-κB-TNF-α Signaling Are Correlated with Nephrectomy-Induced Cognitive Impairment in Rats.
Degaspari S, Tzanno-Martins CB, Fujihara CK, Zatz R, Branco-Martins JP, Viel TA, Buck Hde S, Orellana AM, Böhmer AE, Lima Lde S, Andreotti DZ, Munhoz CD, Scavone C, Kawamoto EM. Degaspari S, et al. PLoS One. 2015 May 11;10(5):e0125271. doi: 10.1371/journal.pone.0125271. eCollection 2015. PLoS One. 2015. PMID: 25961830 Free PMC article. - Association Between Immunoglobulin G _N_-glycosylation and Vascular Cognitive Impairment in a Sample With Atherosclerosis: A Case-Control Study.
Wang M, Chen X, Tang Z, Zhang W, Hou H, Sun X, Shi Y, Lu X, Li P, Ji L, Ding G, Li D. Wang M, et al. Front Aging Neurosci. 2022 Feb 10;14:823468. doi: 10.3389/fnagi.2022.823468. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35221999 Free PMC article. - Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines.
Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, Brenhouse HC. Grassi-Oliveira R, et al. Psychoneuroendocrinology. 2016 Sep;71:19-30. doi: 10.1016/j.psyneuen.2016.04.016. Epub 2016 Apr 30. Psychoneuroendocrinology. 2016. PMID: 27235636 Free PMC article. - AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice.
Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T. Kiyota T, et al. Gene Ther. 2012 Jul;19(7):724-33. doi: 10.1038/gt.2011.126. Epub 2011 Sep 15. Gene Ther. 2012. PMID: 21918553 Free PMC article. - Sex differences and similarities in the neuroimmune response to central administration of poly I:C.
Posillico CK, Garcia-Hernandez RE, Tronson NC. Posillico CK, et al. J Neuroinflammation. 2021 Sep 6;18(1):193. doi: 10.1186/s12974-021-02235-7. J Neuroinflammation. 2021. PMID: 34488804 Free PMC article.
References
- Agnello D, Villa P, Ghezzi P. Increased tumor necrosis factor and interleukin-6 production in the central nervous system of interleukin-10-deficient mice. Brain Res. 2000;869:241–243. - PubMed
- Aubert A, Vega C, Dantzer R, Goodall G. Pyrogens specifically disrupt the acquisition of a task involving cognitive processing in the rat. Brain Behav Immun. 1995;9:129–148. - PubMed
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. - PubMed
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. - PubMed
- Ben Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T, Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–2262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG016710/AG/NIA NIH HHS/United States
- R01 AG016710-10/AG/NIA NIH HHS/United States
- AG 023580/AG/NIA NIH HHS/United States
- R01 AG023580/AG/NIA NIH HHS/United States
- R01 AG023580-05/AG/NIA NIH HHS/United States
- AG 016710/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous