ER membrane-bending proteins are necessary for de novo nuclear pore formation - PubMed (original) (raw)
ER membrane-bending proteins are necessary for de novo nuclear pore formation
T Renee Dawson et al. J Cell Biol. 2009.
Abstract
Nucleocytoplasmic transport occurs exclusively through nuclear pore complexes (NPCs) embedded in pores formed by inner and outer nuclear membrane fusion. The mechanism for de novo pore and NPC biogenesis remains unclear. Reticulons (RTNs) and Yop1/DP1 are conserved membrane protein families required to form and maintain the tubular endoplasmic reticulum (ER) and the postmitotic nuclear envelope. In this study, we report that members of the RTN and Yop1/DP1 families are required for nuclear pore formation. Analysis of Saccharomyces cerevisiae prp20-G282S and nup133 Delta NPC assembly mutants revealed perturbations in Rtn1-green fluorescent protein (GFP) and Yop1-GFP ER distribution and colocalization to NPC clusters. Combined deletion of RTN1 and YOP1 resulted in NPC clustering, nuclear import defects, and synthetic lethality with the additional absence of Pom34, Pom152, and Nup84 subcomplex members. We tested for a direct role in NPC biogenesis using Xenopus laevis in vitro assays and found that anti-Rtn4a antibodies specifically inhibited de novo nuclear pore formation. We hypothesize that these ER membrane-bending proteins mediate early NPC assembly steps.
Figures
Figure 1.
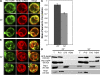
Rtn1-GFP mislocalizes and is associated with an HSM fraction in the prp20-G282S assembly mutant. (A and B) Rtn1-GFP and dsRed-HDEL localization in the prp20-G282S mutant background was visualized by direct laser-scanning confocal microscopy. The prp20-G282S mutant (SWY3747) was grown to early log phase (23°C; A) and shifted to 34°C for 5 h (B). Single-channel fluorescence is shown from three representative cells at each temperature, and merged images are shown (right). (C) Quantitative analysis of relative Rtn1-GFP and dsRed-HDEL distribution in prp20-G282S cells at 23 and 34°C. n = 12 cells for each respective Pearson's coefficient of correlation (+1.0 = complete coincidence; −1.0 = no coincidence). Error bars represent standard deviation. (D) Biochemical fractionation was performed with rtn1-GFP GFP-nic96 prp20-G282S cells (SWY3748) grown to early log phase (23°C) and shifted to 34°C for 5 h. After lysis, subcellular fractionation was performed by differential centrifugation. Cellular equivalent fractions of the total lysate (T), pellet (P; 10,000 g), and supernatant (S; 10,000 g) fractions and 10 times the cellular equivalent of the HSM fraction (135,000-g pellet from the supernatant fraction) were separated by SDS-PAGE, transferred to nitrocellulose, and analyzed by immunoblotting with an anti-GFP. Distributions of cytoplasmic Pgk1 and nucleolar Nop1 were analyzed as controls. Bar, 2.5 µm.
Figure 2.
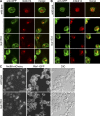
Rtn1-GFP and Yop1-GFP are associated with NPC clusters in the nup133Δ mutant. (A and B) Indirect immunofluorescence microscopy was conducted with parental rtn1-GFP cells (A, top), nup133Δ rtn1-GFP cells (SWY4007; A), parental yop1-GFP cells (B, top), or nup133Δ yop1-GFP cells (SWY4045; B) grown to early log phase at 23°C using rabbit anti-GFP and mouse anti–FG Nup mAb414 antibodies. Direct laser-scanning confocal microscopy was used with single-channel fluorescence for the GFP and mAb414 staining shown from three representative fields. Merged images are shown (right). (C) Live cell direct fluorescence microscopy was conducted with rtn1-GFP nic96-mCherry (SWY4125) and rtn1-GFP nic96-mCherry nup133Δ cells (SWY2117) grown to early log phase at 23°C. DIC, differential interference contrast. Bars: (A and B) 2.5 µm; (C) 10 µm.
Figure 3.
Nups mislocalize in the rtn1Δ yop1Δ mutant. (A) Parental cells (SWY1695 or SWY809) or rtn1Δ yop1Δ cells expressing either GFP-Nic96 or Nup49-GFP (SWY3931 or SWY3880) were grown to early log phase at 23°C and were visualized by direct fluorescence microscopy. (B) Parental cells (BY4742) or rtn1Δ yop1Δ cells (SWY3811) were grown to early log phase at 23°C and processed for indirect immunofluorescence microscopy with anti-Nup116C or anti–Nup116-GLFG antibodies. Nuclei were detected with the DNA dye DAPI. DIC, differential interference contrast. Bars, 10 µm.
Figure 4.
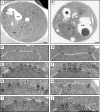
NPCs are clustered and morphologically abnormal in rtn1Δ yop1Δ cells. (A–J) Parental cells (BY4742; A, C, and D) or rtn1Δ yop1Δ cells (SWY3811; B and E–J) were grown to early log phase at 23°C and processed for thin-section EM. Black and white arrows point to the NPC, black arrowheads point to NPC-like structures at the ONM, white arrowheads point to NPC-like structures at the INM, and the asterisks denote other aberrant NPC-like structures at the NE. N, nucleus; C, cytoplasm; vac, vacuole. Bars, 0.5 µm.
Figure 5.
The rtn1Δ yop1Δ mutant has Nab2NLS-GFP nuclear import defects. Parental (BY4742) and rtn1Δ yop1Δ (SWY3811) cells with a plasmid expressing Nab2NLS-GFP (pNS167; Shulga et al., 2000) were grown to early log phase at 23°C. Import kinetics were determined after shifting energy-depleted cells with cytoplasmic Nab2NLS-GFP to glucose-containing media. (A) Localization of Nab2NLS-GFP by direct fluorescence microscopy at time points (minutes) after reinitiating import. (B) Kinetic data for three independent trials. Error bars represent standard deviation. Bar, 10 µm.
Figure 6.
Genetic interactions for the rtn1Δ yop1Δ mutant with mutants in genes encoding Nups, Poms, and ER/NE proteins. (A) Analysis of rtn1Δ yop1Δ nup/pom mutants. Haploid strains with the designated chromosomal deletions and wild-type RTN1, POM, or NUP URA3/CEN plasmid were tested for growth on 5-FOA media (SWY3811 + pRS316, SWY3876 + pSW3354, SWY3877 + pSW1079, SWY3878 + pSW1516, SWY4050 + pSW351, SWY4099 + pSW610, and SWY4106 + pSW3459). (B) Rescue of pom34Δ nup59Δ cells (SWY3139) by exogenous expression of RTN1-GFP or rtn1-K48I–GFP. The pom34Δ nup59Δ parental strain harboring a POM34/URA3 plasmid and LEU2 plasmids for POM34, RTN1-GFP, or rtn1-K48I–GFP were assayed for growth on 5-FOA media (23°C for 6 d). (C) Growth of 5-FOA–resistant pom34Δ nup59Δ isolates expressing POM34, rtn1-GFP, rtn1-K48I–GFP, RTN1, or rtn1-K48I was assessed after serial dilution on YPD.
Figure 7.
Exogenous expression of Ndc1 and Pom152 rescues the rtn1Δ yop1Δ Nup mislocalization phenotype. (A and B) The rtn1Δ yop1Δ GFP-nic96 (SWY3931; A) and the rtn1Δ yop1Δ (SWY3811; B) strains were transformed with plasmids expressing NDC1 (pRS315.NDC1; Chial et al., 1998), POM152 (pSW229), POM34 (pSW1517; Miao et al., 2006), or SNL1 (pSW575; Ho et al., 1998). Direct fluorescence microscopy (A) or indirect immunofluorescence microscopy with anti–Nup116-GLFG antibodies (B) was conducted to evaluate NPC clusters. DIC, differential interference contrast. Bars, 10 µm.
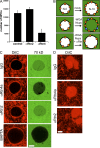
Figure 8.
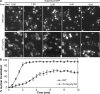
Xenopus Rtn4a is required for de novo NPC formation in vitro. (A) Inhibition of NPC insertion by anti–(α)-Rtn4a but not anti–(α)-Rtn2 antibodies. Preassembled nuclei were incubated with fresh cytosol in the presence of 10 µM of antibodies for 30 min, and pores were visualized by fluorescently labeled WGA. The NPC number of 50 nuclei is plotted. Error bars represent standard deviation. (B) Schematic illustration of dextran influx assay. When preassembled nuclei (N-0) are incubated with mock-depleted cytosol, intact new pores are inserted (orange circles). In contrast, when N-0 nuclei are incubated with WGA-depleted cytosol, new pores form that cannot exclude dextran (blue circles). If RTNs are required for an early step of NPC assembly, pore/hole formation should be blocked and 70-kD dextran should be excluded. (C) Nuclei were assembled with DiIC16(3)-labeled membranes (red) and incubated with WGA-depleted cytosol in the absence (IgG and α-Rtn2) or presence of inhibitory antibodies (α-Rtn4a) or the calcium chelator BAPTA. After a 10-min incubation, fluorescently labeled 70-kD dextran was added (green), and nuclei were immediately imaged using confocal microscopy (n ≥ 20). (D) In the same reactions as C, surface images were taken from unfixed nuclei that did not exclude (IgG and α-Rtn2) or did exclude (α-Rtn4a) 70-kD dextran. Under all conditions, the NEs were visible as flat membrane patches connected to intact ER tubules. Bars, 10 µm.
Similar articles
- Integrity and function of the Saccharomyces cerevisiae spindle pole body depends on connections between the membrane proteins Ndc1, Rtn1, and Yop1.
Casey AK, Dawson TR, Chen J, Friederichs JM, Jaspersen SL, Wente SR. Casey AK, et al. Genetics. 2012 Oct;192(2):441-55. doi: 10.1534/genetics.112.141465. Epub 2012 Jul 13. Genetics. 2012. PMID: 22798490 Free PMC article. - Pom33, a novel transmembrane nucleoporin required for proper nuclear pore complex distribution.
Chadrin A, Hess B, San Roman M, Gatti X, Lombard B, Loew D, Barral Y, Palancade B, Doye V. Chadrin A, et al. J Cell Biol. 2010 May 31;189(5):795-811. doi: 10.1083/jcb.200910043. Epub 2010 May 24. J Cell Biol. 2010. PMID: 20498018 Free PMC article. - The Ran GTPase cycle is required for yeast nuclear pore complex assembly.
Ryan KJ, McCaffery JM, Wente SR. Ryan KJ, et al. J Cell Biol. 2003 Mar 31;160(7):1041-53. doi: 10.1083/jcb.200209116. Epub 2003 Mar 24. J Cell Biol. 2003. PMID: 12654904 Free PMC article. - Structural analysis of the nuclear pore complex by integrated approaches.
Elad N, Maimon T, Frenkiel-Krispin D, Lim RY, Medalia O. Elad N, et al. Curr Opin Struct Biol. 2009 Apr;19(2):226-32. doi: 10.1016/j.sbi.2009.02.009. Epub 2009 Mar 25. Curr Opin Struct Biol. 2009. PMID: 19327984 Review. - Nuclear pore biogenesis into an intact nuclear envelope.
Doucet CM, Hetzer MW. Doucet CM, et al. Chromosoma. 2010 Oct;119(5):469-77. doi: 10.1007/s00412-010-0289-2. Epub 2010 Aug 19. Chromosoma. 2010. PMID: 20721671 Review.
Cited by
- Rapid, optimized interactomic screening.
Hakhverdyan Z, Domanski M, Hough LE, Oroskar AA, Oroskar AR, Keegan S, Dilworth DJ, Molloy KR, Sherman V, Aitchison JD, Fenyö D, Chait BT, Jensen TH, Rout MP, LaCava J. Hakhverdyan Z, et al. Nat Methods. 2015 Jun;12(6):553-60. doi: 10.1038/nmeth.3395. Epub 2015 May 4. Nat Methods. 2015. PMID: 25938370 Free PMC article. - The nuclear pore complex--structure and function at a glance.
Kabachinski G, Schwartz TU. Kabachinski G, et al. J Cell Sci. 2015 Feb 1;128(3):423-9. doi: 10.1242/jcs.083246. J Cell Sci. 2015. PMID: 26046137 Free PMC article. Review. - ER structure and function.
Chen S, Novick P, Ferro-Novick S. Chen S, et al. Curr Opin Cell Biol. 2013 Aug;25(4):428-33. doi: 10.1016/j.ceb.2013.02.006. Epub 2013 Mar 13. Curr Opin Cell Biol. 2013. PMID: 23478217 Free PMC article. Review. - Reticulons Regulate the ER Inheritance Block during ER Stress.
Piña FJ, Fleming T, Pogliano K, Niwa M. Piña FJ, et al. Dev Cell. 2016 May 9;37(3):279-88. doi: 10.1016/j.devcel.2016.03.025. Epub 2016 Apr 21. Dev Cell. 2016. PMID: 27117666 Free PMC article. - Changes in the nuclear envelope environment affect spindle pole body duplication in Saccharomyces cerevisiae.
Witkin KL, Friederichs JM, Cohen-Fix O, Jaspersen SL. Witkin KL, et al. Genetics. 2010 Nov;186(3):867-83. doi: 10.1534/genetics.110.119149. Epub 2010 Aug 16. Genetics. 2010. PMID: 20713690 Free PMC article.
References
- Abramoff M., Magelhaes P., Ram S. 2004. Image processing with ImageJ.Biophotonics International. 11:36–42
- Aitchison J.D., Rout M.P., Marelli M., Blobel G., Wozniak R.W. 1995. Two novel related yeast nucleoporins Nup170p and Nup157p: complementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p.J. Cell Biol. 131:1133–1148 - PMC - PubMed
- Alber F., Dokudovskaya S., Veenhoff L.M., Zhang W., Kipper J., Devos D., Suprapto A., Karni-Schmidt O., Williams R., Chait B.T., et al. 2007a. Determining the architectures of macromolecular assemblies.Nature. 450:683–694 - PubMed
- Alber F., Dokudovskaya S., Veenhoff L.M., Zhang W., Kipper J., Devos D., Suprapto A., Karni-Schmidt O., Williams R., Chait B.T., et al. 2007b. The molecular architecture of the nuclear pore complex.Nature. 450:695–701 - PubMed
- Anderson D.J., Hetzer M.W. 2007. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum.Nat. Cell Biol. 9:1160–1166 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM057438/GM/NIGMS NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- R01 GM57438/GM/NIGMS NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases